Seroepidemiology of enterovirus A71 infection in prospective cohort studies of children in southern China, 2013-2018
- PMID: 36435844
- PMCID: PMC9701185
- DOI: 10.1038/s41467-022-34992-1
Seroepidemiology of enterovirus A71 infection in prospective cohort studies of children in southern China, 2013-2018
Abstract
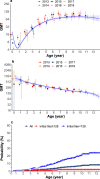
Enterovirus A71 (EV-A71)-related hand, foot, and mouth disease (HFMD) imposes a substantial clinical burden in the Asia Pacific region. To inform policy on the introduction of the EV-A71 vaccine into the National Immunization Programme, we investigated the seroepidemiological characteristics of EV-A71 in two prospective cohorts of children in southern China conducted between 2013 and 2018. Our results show that maternal antibody titres declined rapidly in neonates, with over half becoming susceptible to EV-A71 at 1 month of age. Between 6 months and 2 years of age, over 80% of study participants were susceptible, while one third remained susceptible at 5 years old. The highest incidence of EV-A71 infections was observed in children aged 5-6 months. Our findings support EV-A71 vaccination before 6 months for birth cohorts in southern China, potentially with a one-time catch-up vaccination for children 6 months-5 years old. More regionally representative longitudinal seroepidemiological studies are needed to further validate these findings.
© 2022. The Author(s).
Conflict of interest statement
H.Y. has received investigator-initiated research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, Shanghai Roche Pharmaceutical Company and SINOVAC Biotech Ltd. None of the research funding is related to this study. All other authors report no competing interests.
Figures


Similar articles
-
The transfer and decay of maternal antibodies against enterovirus A71, and dynamics of antibodies due to later natural infections in Chinese infants: a longitudinal, paired mother-neonate cohort study.Lancet Infect Dis. 2021 Mar;21(3):418-426. doi: 10.1016/S1473-3099(20)30480-1. Epub 2020 Oct 5. Lancet Infect Dis. 2021. PMID: 33031750
-
[Analysis on epidemiological characteristics of enterovirus 71 cases of hand-foot-mouth disease based on the active monitoring in Guangdong Province in 2011-2015].Zhonghua Yu Fang Yi Xue Za Zhi. 2018 Jul 6;52(7):738-742. doi: 10.3760/cma.j.issn.0253-9624.2018.07.011. Zhonghua Yu Fang Yi Xue Za Zhi. 2018. PMID: 29996302 Chinese.
-
Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination.PLoS Med. 2016 Feb 16;13(2):e1001958. doi: 10.1371/journal.pmed.1001958. eCollection 2016 Feb. PLoS Med. 2016. PMID: 26882540 Free PMC article.
-
Enterovirus A71: virulence, antigenicity, and genetic evolution over the years.J Biomed Sci. 2019 Oct 21;26(1):81. doi: 10.1186/s12929-019-0574-1. J Biomed Sci. 2019. PMID: 31630680 Free PMC article. Review.
-
Establishment of Asia-Pacific Network for Enterovirus Surveillance.Vaccine. 2020 Jan 3;38(1):1-9. doi: 10.1016/j.vaccine.2019.09.111. Epub 2019 Oct 31. Vaccine. 2020. PMID: 31679864 Review.
Cited by
-
Dynamics of measles immunity from birth and following vaccination.Nat Microbiol. 2024 Jul;9(7):1676-1685. doi: 10.1038/s41564-024-01694-x. Epub 2024 May 13. Nat Microbiol. 2024. PMID: 38740931
-
Increasing intensity of enterovirus outbreaks projected with climate change.Nat Commun. 2024 Jul 31;15(1):6466. doi: 10.1038/s41467-024-50936-3. Nat Commun. 2024. PMID: 39085256 Free PMC article.
-
Enterovirus A71 priorities, challenges, and future opportunities in humoral immunity and vaccine development.NPJ Vaccines. 2025 Aug 15;10(1):194. doi: 10.1038/s41541-025-01184-z. NPJ Vaccines. 2025. PMID: 40817335 Free PMC article. Review.
-
Age-time-specific transmission of hand-foot-and-mouth disease enterovirus serotypes in Vietnam: A catalytic model with maternal immunity.Epidemics. 2024 Mar;46:100754. doi: 10.1016/j.epidem.2024.100754. Epub 2024 Feb 27. Epidemics. 2024. PMID: 38428358 Free PMC article.
-
Epidemiology of Enterovirus Genotypes in Association with Human Diseases.Viruses. 2024 Jul 19;16(7):1165. doi: 10.3390/v16071165. Viruses. 2024. PMID: 39066327 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

