Mechanisms of colorectal liver metastasis development
- PMID: 36436127
- PMCID: PMC9701652
- DOI: 10.1007/s00018-022-04630-6
Mechanisms of colorectal liver metastasis development
Abstract
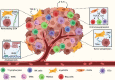
Colorectal cancer (CRC) is a leading cause of cancer-related death worldwide, largely due to the development of colorectal liver metastases (CRLM). For the establishment of CRLM, CRC cells must remodel their tumor-microenvironment (TME), avoid the immune system, invade the underlying stroma, survive the hostile environment of the circulation, extravasate into the liver, reprogram the hepatic microenvironment into a permissive pre-metastatic niche, and finally, awake from a dormant state to grow out into clinically detectable CRLM. These steps form part of the invasion-metastasis cascade that relies on reciprocal interactions between the tumor and its ever-changing microenvironment. Such interplay provides a strong rational for therapeutically targeting the TME. In fact, several TME constituents, such as VEGF, TGF-β coreceptor endoglin, and CXCR4, are already targeted in clinical trials. It is, however, of utmost importance to fully understand the complex interactions in the invasion-metastasis cascade to identify novel potential therapeutic targets and prevent the establishment of CRLM, which may ultimately greatly improve patient outcome.
Keywords: Cancer; Circulating tumor cells; Epithelial-mesenchymal transition; Invasion-metastasis cascade; Pre-metastatic niche; Tumor microenvironment.
© 2022. The Author(s).
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures


References
-
- Manfredi S, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

