Ustekinumab and Vedolizumab Are Equally Safe and Effective in Elderly Crohn's Disease Patients
- PMID: 36436155
- PMCID: PMC9702964
- DOI: 10.1007/s10620-022-07770-8
Ustekinumab and Vedolizumab Are Equally Safe and Effective in Elderly Crohn's Disease Patients
Abstract
Background: Anti-tumour necrosis factor (anti-TNF) agents are associated with increased infection risk among elderly inflammatory bowel disease (IBD) patients, and thus, alternative biologics may be preferable. However, little comparative data exist on the safety and efficacy of vedolizumab and ustekinumab in elderly IBD patients.
Aims: To compare the safety and effectiveness of ustekinumab and vedolizumab in elderly Crohn's disease patients.
Methods: Patients ≥ 60 years old who commenced ustekinumab or vedolizumab for Crohn's disease (CD) were included. Primary outcome was serious infections, defined as requiring hospitalisation. Efficacy was assessed by treatment persistence and clinical response rates. We appropriately adjusted for confounders using propensity score-matched analysis weighted by the inverse predicted probability of treatment weighing and performed a logistic regression analysis to assess factors associated with serious infections and treatment persistence.
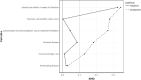
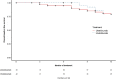
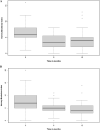
Results: Eighty-three patients commencing ustekinumab and 42 commencing vedolizumab therapy were included. In a propensity adjusted cohort, the rate of serious infection and treatment persistence were comparable between ustekinumab and vedolizumab. There was a significant reduction in HBI at 6 and 12 months compared to baseline in both groups. Male gender was positively associated with serious infection risk at 12 months, and penetrating disease behaviour was positively associated with 12-month treatment persistence. Baseline HBI score was negatively associated with 12-month treatment persistence. Cox regression analysis showed no overall difference in treatment discontinuation-free and serious infection-free survival by 12 months.
Conclusions: We observed comparable safety and effectiveness for ustekinumab and vedolizumab in treating elderly CD patients.
Keywords: Crohn’s disease; Effectiveness; Elderly; Safety; Ustekinumab; Vedolizumab.
© 2022. Crown.
Conflict of interest statement
GGG, JF, EL, GB and VR report no conflicts of interest. JKL has received speaker fees from Dr. Falk pharmaceuticals. SS has received speaker fees from Abbvie, Dr. Falk pharmaceuticals, Takeda, Janssen, Celltrion, Bristol-Myers Squibb and received educational grants from Abbvie, Takeda and Janssen and is an advisory board member for Abbvie, Dr. Falk pharmaceuticals, Vifor pharmaceuticals, Janssen, Takeda and Celltrion. SS is an associate editor for AP&T. PKF received a Shire Innovation Fund award and has received funded conference travel from Shire and Tillotts. PJS has received speaker fees from Takeda, Janssen, Celltrion, Abbvie, Amgen, Dr. Falk pharmaceuticals, Tillotts Pharma and has been an advisory board member for Abbvie, Celltrion and Janssen. EL has received speaker fees from Janssen and a research grant from Galapagos. CPS has received unrestricted research grants from Abbvie and Janssen, has provided consultancy to Arena, Galapagos, Dr. Falk, Fresenius Kabi, Abbvie, Janssen and Takeda, and had speaker arrangements with Janssen, Dr. Falk, Abbvie, Pfizer and Takeda. All authors approve the final version of the manuscript.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

