Transcriptional pathways linked to fetal and maternal hepatic dysfunction caused by gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) in CD-1 mice
- PMID: 36436258
- PMCID: PMC9742811
- DOI: 10.1016/j.ecoenv.2022.114314
Transcriptional pathways linked to fetal and maternal hepatic dysfunction caused by gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) in CD-1 mice
Abstract
Per- and polyfluoroalkyl substances (PFAS) comprise a diverse class of chemicals used in industrial processes, consumer products, and fire-fighting foams which have become environmental pollutants of concern due to their persistence, ubiquity, and associations with adverse human health outcomes, including in pregnant persons and their offspring. Multiple PFAS are associated with adverse liver outcomes in adult humans and toxicological models, but effects on the developing liver are not fully described. Here we performed transcriptomic analyses in the mouse to investigate the molecular mechanisms of hepatic toxicity in the dam and its fetus after exposure to two different PFAS, perfluorooctanoic acid (PFOA) and its replacement, hexafluoropropylene oxide-dimer acid (HFPO-DA, known as GenX). Pregnant CD-1 mice were exposed via oral gavage from embryonic day (E) 1.5-17.5 to PFOA (0, 1, or 5 mg/kg-d) or GenX (0, 2, or 10 mg/kg-d). Maternal and fetal liver RNA was isolated (N = 5 per dose/group) and the transcriptome analyzed by Affymetrix Array. Differentially expressed genes (DEG) and differentially enriched pathways (DEP) were obtained. DEG patterns were similar in maternal liver for 5 mg/kg PFOA, 2 mg/kg GenX, and 10 mg/kg GenX (R2: 0.46-0.66). DEG patterns were similar across all 4 dose groups in fetal liver (R2: 0.59-0.81). There were more DEGs in fetal liver compared to maternal liver at the low doses for both PFOA (fetal = 69, maternal = 8) and GenX (fetal = 154, maternal = 93). Upregulated DEPs identified across all groups included Fatty Acid Metabolism, Peroxisome, Oxidative Phosphorylation, Adipogenesis, and Bile Acid Metabolism. Transcriptome-phenotype correlation analyses demonstrated > 1000 maternal liver DEGs were significantly correlated with maternal relative liver weight (R2 >0.92). These findings show shared biological pathways of liver toxicity for PFOA and GenX in maternal and fetal livers in CD-1 mice. The limited overlap in specific DEGs between the dam and fetus suggests the developing liver responds differently than the adult liver to these chemical stressors. This work helps define mechanisms of hepatic toxicity of two structurally unique PFAS and may help predict latent consequences of developmental exposure.
Keywords: Animal models; Developmental exposure; Emerging contaminants; Liver disease; PFAS.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
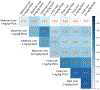

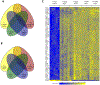



References
-
- Abbott BD, Wood CR, Watkins AM, Tatum-Gibbs K, Das KP, Lau C, 2012. Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and postnatal CD-1 mouse tissues. Reprod. Toxicol 33 (4), 491–505, 1. - PubMed
-
- ATSDR Toxicological Profile for Perfluoroalkyls Agency for Toxic Substances and Disease Registry 2020.https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. - PubMed
-
- Bauman DE, Currie WB, 1980. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci 63, 1514–1529. - PubMed
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

