Genotype-first approach to identify associations between CDH1 germline variants and cancer phenotypes: a multicentre study by the European Reference Network on Genetic Tumour Risk Syndromes
- PMID: 36436516
- PMCID: PMC9810541
- DOI: 10.1016/S1470-2045(22)00643-X
Genotype-first approach to identify associations between CDH1 germline variants and cancer phenotypes: a multicentre study by the European Reference Network on Genetic Tumour Risk Syndromes
Erratum in
-
Correction to Lancet Oncol 2023; 24: 91-106.Lancet Oncol. 2023 Jan;24(1):e10. doi: 10.1016/S1470-2045(22)00761-6. Lancet Oncol. 2023. PMID: 36603924 Free PMC article. No abstract available.
Abstract
Background: Truncating pathogenic or likely pathogenic variants of CDH1 cause hereditary diffuse gastric cancer (HDGC), a tumour risk syndrome that predisposes carrier individuals to diffuse gastric and lobular breast cancer. Rare CDH1 missense variants are often classified as variants of unknown significance. We conducted a genotype-phenotype analysis in families carrying rare CDH1 variants, comparing cancer spectrum in carriers of pathogenic or likely pathogenic variants (PV/LPV; analysed jointly) or missense variants of unknown significance, assessing the frequency of families with lobular breast cancer among PV/LPV carrier families, and testing the performance of lobular breast cancer-expanded criteria for CDH1 testing.
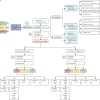
Methods: This genotype-first study used retrospective diagnostic and clinical data from 854 carriers of 398 rare CDH1 variants and 1021 relatives, irrespective of HDGC clinical criteria, from 29 institutions in ten member-countries of the European Reference Network on Tumour Risk Syndromes (ERN GENTURIS). Data were collected from Oct 1, 2018, to Sept 20, 2022. Variants were classified by molecular type and clinical actionability with the American College of Medical Genetics and Association for Molecular Pathology CDH1 guidelines (version 2). Families were categorised by whether they fulfilled the 2015 and 2020 HDGC clinical criteria. Genotype-phenotype associations were analysed by Student's t test, Kruskal-Wallis, χ2, and multivariable logistic regression models. Performance of HDGC clinical criteria sets were assessed with an equivalence test and Youden index, and the areas under the receiver operating characteristic curves were compared by Z test.
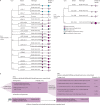
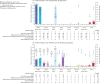
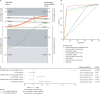
Findings: From 1971 phenotypes (contributed by 854 probands and 1021 relatives aged 1-93 years), 460 had gastric and breast cancer histology available. CDH1 truncating PV/LPVs occurred in 176 (21%) of 854 families and missense variants of unknown significance in 169 (20%) families. Multivariable logistic regression comparing phenotypes occurring in families carrying PV/LPVs or missense variants of unknown significance showed that lobular breast cancer had the greatest positive association with the presence of PV/LPVs (odds ratio 12·39 [95% CI 2·66-57·74], p=0·0014), followed by diffuse gastric cancer (8·00 [2·18-29·39], p=0·0017) and gastric cancer (7·81 [2·03-29·96], p=0·0027). 136 (77%) of 176 families carrying PV/LPVs fulfilled the 2015 HDGC criteria. Of the remaining 40 (23%) families, who did not fulfil the 2015 criteria, 11 fulfilled the 2020 HDGC criteria, and 18 had lobular breast cancer only or lobular breast cancer and gastric cancer, but did not meet the 2020 criteria. No specific CDH1 variant was found to predispose individuals specifically to lobular breast cancer, although 12 (7%) of 176 PV/LPV carrier families had lobular breast cancer only. Addition of three new lobular breast cancer-centred criteria improved testing sensitivity while retaining high specificity. The probability of finding CDH1 PV/LPVs in patients fulfilling the lobular breast cancer-expanded criteria, compared with the 2020 criteria, increased significantly (AUC 0·92 vs 0·88; Z score 3·54; p=0·0004).
Interpretation: CDH1 PV/LPVs were positively associated with HDGC-related phenotypes (lobular breast cancer, diffuse gastric cancer, and gastric cancer), and no evidence for a positive association with these phenotypes was found for CDH1 missense variants of unknown significance. CDH1 PV/LPVs occurred often in families with lobular breast cancer who did not fulfil the 2020 HDGC criteria, supporting the expansion of lobular breast cancer-centred criteria.
Funding: European Reference Network on Genetic Tumour Risk Syndromes, European Regional Development Fund, Fundação para a Ciência e a Tecnologia (Portugal), Cancer Research UK, and European Union's Horizon 2020 research and innovation programme.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DGE declares fees from Astrazeneca and Recursion. ERW declares grants from International Alliance for Cancer Early Detection, for which they are codirector of the research domain. GNR declares receipt of funding for study materials, medical writing, and article processing charges from Italian Ministry of Education (GNR). MJLL declares consulting fees (via the Radboud University Medical Center) from Merck Sharp & Dohme (MSD), AstraZeneca, Lilly, Janssen-Cilag, Illumina, GlaxoSmithKline. PRB declares fees from AstraZeneca, MSD, and Bristol Myers Squibb; and is a scientific committee member for the Geneticancer patients association. JBa declares fees from AstraZeneca, Lilly, and Pfizer. SA is a member of APC subVCEP of the InSiGHT/ClinGen Hereditary Colorectal Cancer/Polyposis Variant Curation Expert Panel; is an unpaid member of the German Gene Diagnostics Commission and speaker of the Centre for Hereditary Tumour Syndromes of the University of Bonn. RH declares grants from SLA Pharma and Janssen Pharmaceuticals; consulting fees from Janssen and One Two Therapeutics; equipment from Fujifilm; is the head of German Consortium for Familial Gastrointestinal Cancer; and is an unpaid advisory board member of the Lynch Syndrome advocacy Group and the Familial Polyposis Group. ES declares grants from NCT/DKTK Master. ES declares honoraria for presentations from AstraZeneca, Georg Thieme Verlag KG, and payment for expert testimony from Illumina; is a member of the board of directors of Deutsche Gesellschaft für HumanGenetik; an advisor for Dresden-concept Genome Center; and is board of directors president (paid) for LNS laboratoire National de Santé. RdP declares support for presentations (via his institution) from MSD and AstraZeneca. GC declares to receive funding for study materials, medical writing, article processing charges from the Spanish Ministry of Science and Innovation, the Instituto de Salud Carlos III CIBERONC, and the Government of Catalonia.; consulting fees from VCN Biosciences Synthetic Biologics; is the chair of the Council of the International Society of Hereditary Gastrointestinal Tumours and the FUREGA Fundació Recerca en Gastroenterologia; and stock in Synthetic Biologics. CLa declares consulting fees and honoraria from AstraZeneca and MSD, and is a paid advisory board member for Illumina.
Figures



References
-
- Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. - PubMed

