Glucose transporter GLUT1 expression is important for oriental river prawn (Macrobrachium nipponense) hemocyte adaptation to hypoxic conditions
- PMID: 36436564
- PMCID: PMC9758439
- DOI: 10.1016/j.jbc.2022.102748
Glucose transporter GLUT1 expression is important for oriental river prawn (Macrobrachium nipponense) hemocyte adaptation to hypoxic conditions
Abstract
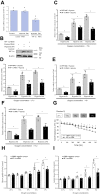
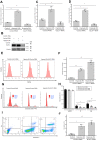
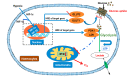
Crustaceans have an open vascular system in which hemocytes freely circulate in hemolymph. Hemocytes are rich in hemocyanin, a specific oxygen-transport protein in crustaceans; therefore, understanding the response of hemocytes to hypoxia is crucial. Although hemocytes take up glucose during hypoxia, the molecular mechanism of glucose uptake in crustaceans remains unclear. Herein, we identified two highly conserved glucose transporters (GLUT1 and GLUT2) in Macrobrachium nipponense (oriental river prawn) and analyzed their tissue-specific expression patterns. Our immunofluorescence assays showed that GLUT1 and GLUT2 are located on the cell membrane, with a strong GLUT1 signal in primary hemocytes under hypoxia. We found that during acute hypoxia, hypoxia-inducible factor-1α-related metabolic alterations result in decreased mitochondrial cytochrome c oxidase activity, implying a classic glycolytic mechanism. As a proof of concept, we replicated these findings in insect S2 cells. Acute hypoxia significantly induced hypoxia-inducible factor-1α, GLUT1, and pyruvate dehydrogenase kinase isozyme 1 expression in primary hemocytes, and hypoxia-induced increases in glucose uptake and lactate secretion were observed. GLUT1 knockdown induced intracellular reactive oxygen species generation and apoptosis in vitro and in vivo, resulting in increased prawn mortality and more apoptotic cells in their brains, implying a vital function of GLUT1 in hypoxia adaptation. Taken together, our results suggest a close relationship between hypoxia-mediated glycolysis and GLUT1 in hemocytes. These results demonstrated that in crustaceans, adaptation to hypoxia involves glucose metabolic plasticity.
Keywords: GLUT1; glucose transport; glycolysis; hypoxia; invertebrate.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Hypoxia induces ferroptotic cell death mediated by activation of the inner mitochondrial membrane fission protein MTP18/Drp1 in invertebrates.J Biol Chem. 2025 Mar;301(3):108326. doi: 10.1016/j.jbc.2025.108326. Epub 2025 Feb 18. J Biol Chem. 2025. PMID: 39971157 Free PMC article.
-
Differential regulation of GLUT1 and GLUT8 expression by hypoxia in mammary epithelial cells.Am J Physiol Regul Integr Comp Physiol. 2014 Aug 1;307(3):R237-47. doi: 10.1152/ajpregu.00093.2014. Epub 2014 Jun 11. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24920730
-
Molecular Cloning and Expression Analysis of Lactate Dehydrogenase from the Oriental River Prawn Macrobrachium nipponense in Response to Hypoxia.Int J Mol Sci. 2018 Jul 8;19(7):1990. doi: 10.3390/ijms19071990. Int J Mol Sci. 2018. PMID: 29986527 Free PMC article.
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
-
[The role of glucose transporter 1 (GLUT1) in the diagnosis and therapy of tumors].Postepy Hig Med Dosw (Online). 2012 Jan 4;66:165-74. doi: 10.5604/17322693.988242. Postepy Hig Med Dosw (Online). 2012. PMID: 22470192 Review. Polish.
Cited by
-
Hypoxia induces ferroptotic cell death mediated by activation of the inner mitochondrial membrane fission protein MTP18/Drp1 in invertebrates.J Biol Chem. 2025 Mar;301(3):108326. doi: 10.1016/j.jbc.2025.108326. Epub 2025 Feb 18. J Biol Chem. 2025. PMID: 39971157 Free PMC article.
References
-
- Rytkönen K.T., Vuori K.A.M., Primmer C.R., Nikinmaa M. Comparison of hypoxia-inducible factor-1alpha in hypoxia-sensitive and hypoxia-tolerant fish species. Comp. Biochem. Physiol. Part D Genom. Proteom. 2007;2:177–186. - PubMed
-
- Wu R.S.S. Hypoxia: from molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002;45:35–45. - PubMed
-
- Wu R.S., Zhou B.S., Randall D.J., Woo N.Y., Lam P.K. Aquatic hypoxia is an endocrine disruptor and impairs fish reproduction. Environ. Sci. Technol. 2003;37:1137–1141. - PubMed
-
- Abdel-Tawwab M., Monier M.N., Hoseinifar S.H., Faggio C. Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019;45:997–1013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

