Inflammatory bowel disease-specific findings are common morphological changes in the ileal pouch with ulcerative colitis
- PMID: 36437274
- PMCID: PMC9701810
- DOI: 10.1038/s41598-022-24708-2
Inflammatory bowel disease-specific findings are common morphological changes in the ileal pouch with ulcerative colitis
Abstract
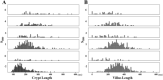
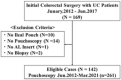
Why inflammation is common in ileal pouches with ulcerative colitis (UC) is unclear. We therefore clarified the morphological changes in pouches and afferent limbs (AL) of patients with UC and explored the relationship between these findings. We evaluated the morphological findings (histological and endoscopic inflammation as the Pouchitis Disease Activity Index [PDAI] histology subscore [hPDAI] and endoscopy subscore [ePDAI], inflammatory bowel disease [IBD]-specific findings using the IBD score [SIBD], colonic metaplasia using the colonic metaplasia score [CMS], and goblet cell [GC] ratio) in the pouch and AL of patients with UC. A total of 261 pouchoscopies were analyzed. The pouch body had a higher hPDAI (p < 0.001), SIBD (p < 0.001), CMS (p < 0.001), GC ratio (p < 0.001), and ePDAI (p < 0.001) than the AL. The hPDAI was correlated with the SIBD (Spearman's coefficient r = 0.538; p < 0.001), CMS (r = 0.687; p < 0.001), and the ePDAI (r = 0.552; p < 0.001), but not with GC ratio (r = 0.175; p < 0.001) or the pouch usage duration (r = -0.057; p = 0.107). The incidence of histological inflammation was higher in specimens showing basal plasmacytosis with severe mononuclear cell infiltration (BP) than in those without BP (odds ratio [OR] 6.790, p < 0.001), BP was commonly found with crypt hyperplasia (OR 3.414, p < 0.001) and the crypt length correlated with neutrophil infiltration (r = 0.469; p < 0.001). Histological inflammation, colonic metaplasia, the GC ratio, endoscopic inflammation, and IBD-specific findings were commonly present in the pouch than in the AL. Histological inflammation occurs with IBD-specific findings and colonic metaplasia, and these signify endoscopic inflammation.
© 2022. The Author(s).
Conflict of interest statement
Hideaki Kimura reports receiving personal fees from Mitsubishi Tanabe Pharma, EA Pharma, ZERIA Pharmaceuticals and Janssen Pharmaceuticals while conducting the study. Reiko Kunisaki reports receiving research grants or personal fees from AbbVie GK, Janssen Pharmaceutical K.K., JIMRO Co,. Ltd, Kyorin Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Nippon Kayaku Co.,Ltd, Mitsubishi Tanabe Pharma Corporation, Takeda Pharma Co Ltd and Zeria Pharmaceutical Co. Ltd while conducting the study. Jun Watanabe reports receiving honoraria for lectures from Johnson and Johnson, Medtronic and Eli Lilly, and receiving research funding from Medtronic, AMCO and TERUMO outside the submitted work. Itaru Endo reports receiving personal fees from ASAHI KASEI PHARMA CORPORATION while conducting the study. Kenichiro Toritani, Masako Otani, Hironori Fukuoka, Atsushi Ishibe, Toshihiro Misumi, and Yoshiaki Inayama have no financial disclosures or conflicts of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

