FGF-23 protects cell function and viability in murine pancreatic islets challenged by glucolipotoxicity
- PMID: 36437429
- PMCID: PMC9908675
- DOI: 10.1007/s00424-022-02772-x
FGF-23 protects cell function and viability in murine pancreatic islets challenged by glucolipotoxicity
Abstract
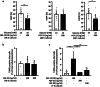
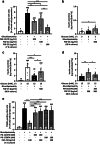
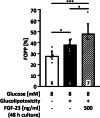
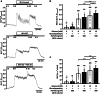
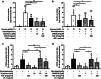
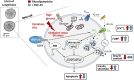
The fibroblast growth factor FGF-23 is a member of the FGF-15/19 subfamily with hormonal functions. Besides its well-known role for bone mineralization, FGF-23 is discussed as a marker for cardiovascular disease. We investigated whether FGF-23 has any effects on the endocrine pancreas of mice by determining insulin secretion, electrical activity, intracellular Ca2+, and apoptosis. Acute application of FGF-23 (10 to 500 ng/ml, i.e., 0.4 to 20 nM) does not affect insulin release of murine islets, while prolonged exposure leads to a 21% decrease in glucose-stimulated secretion. The present study shows for the first time that FGF-23 (100 or 500 ng/ml) partially protects against impairment of insulin secretion and apoptotic cell death induced by glucolipotoxicity. The reduction of apoptosis by FGF-23 is approximately twofold higher compared to FGF-21 or FGF-15/19. In contrast to FGF-23 and FGF-21, FGF-15/19 is clearly pro-apoptotic under control conditions. The beneficial effect of FGF-23 against glucolipotoxicity involves interactions with the stimulus-secretion cascade of beta-cells. Electrical activity and the rise in the cytosolic Ca2+ concentration of islets in response to acute glucose stimulation increase after glucolipotoxic culture (48 h). Co-culture with FGF-23 further elevates the glucose-mediated effects on both parameters. Protection against apoptosis and glucolipotoxic impairment of insulin release by FGF-23 is prevented, when calcineurin is inhibited by tacrolimus or when c-Jun N-terminal kinase (JNK) is blocked by SP600125. In conclusion, our data suggest that FGF-23 can activate compensatory mechanisms to maintain beta-cell function and integrity of islets of Langerhans during excessive glucose and lipid supply.
Keywords: Beta-cell; Calcineurin; FGF-23; Glucolipotoxicity; Insulin; JNK.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

