S1P receptor modulators and the cardiovascular autonomic nervous system in multiple sclerosis: a narrative review
- PMID: 36437849
- PMCID: PMC9685213
- DOI: 10.1177/17562864221133163
S1P receptor modulators and the cardiovascular autonomic nervous system in multiple sclerosis: a narrative review
Abstract
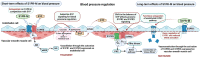
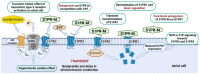
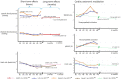
Sphingosine 1-phosphate (S1P) receptor (S1PR) modulators have a complex mechanism of action, which are among the most efficient therapeutic options in multiple sclerosis (MS) and represent a promising approach for other immune-mediated diseases. The S1P signaling pathway involves the activation of five extracellular S1PR subtypes (S1PR1-S1PR5) that are ubiquitous and have a wide range of effects. Besides the immunomodulatory beneficial outcome in MS, S1P signaling regulates the cardiovascular function via S1PR1-S1PR3 subtypes, which reside on cardiac myocytes, endothelial, and vascular smooth muscle cells. In our review, we describe the mechanisms and clinical effects of S1PR modulators on the cardiovascular system. In the past, mostly short-term effects of S1PR modulators on the cardiovascular system have been studied, while data on long-term effects still need to be investigated. Immediate effects detected after treatment initiation are due to parasympathetic overactivation. In contrast, long-term effects may arise from a shift of the autonomic regulation toward sympathetic predominance along with S1PR1 downregulation. A mild increase in blood pressure has been reported in long-term studies, as well as decreased baroreflex sensitivity. In most studies, sustained hypertension was found to represent a significant adverse event related to treatment. The shift in the autonomic control and blood pressure values could not be just a consequence of disease progression but also related to S1PR modulation. Reduced cardiac autonomic activation and decreased heart rate variability during the long-term treatment with S1PR modulators may increase the risk for subsequent cardiac events. For second-generation S1PR modulators, this observation has to be confirmed in further studies with longer follow-ups. The periodic surveillance of cardiovascular function and detection of any cardiac autonomic dysfunction can help predict cardiac outcomes not only after the first dose but also throughout treatment.
Plain language summary: What is the cardiovascular effect of S1P receptor modulator therapy in multiple sclerosis? Sphingosine 1-phosphate (S1P) receptor (S1PR) modulators are among the most efficient therapies for multiple sclerosis. As small molecules, they are not only acting on the immune but on cardiovascular and nervous systems as well. Short-term effects of S1PR modulators on the cardiovascular system have already been extensively described, while long-term effects are less known. Our review describes the mechanisms of action and the short- and long-term effects of these therapeutic agents on the cardiovascular system in different clinical trials. We systematically reviewed the literature that had been published by January 2022. One hundred seven articles were initially identified by title and abstract using targeted keywords, and thirty-nine articles with relevance to cardiovascular effects of S1PR therapy in multiple sclerosis patients were thereafter considered, including their references for further accurate clarification. Studies on fingolimod, the first S1PR modulator approved for treating multiple sclerosis, primarily support the safety profile of this therapeutic class. The second-generation therapeutic agents along with a different treatment initiation approach helped mitigate several of the cardiovascular adverse effects that had previously been observed at the start of treatment. The heart rate may decrease when initiating S1PR modulators and, less commonly, the atrioventricular conduction may be prolonged, requiring cardiac monitoring for the first 6 h of medication. Continuous therapy with S1PR modulators can increase blood pressure values; therefore, the presence of arterial hypertension should be checked during long-term treatment. Periodic surveillance of the cardiovascular and autonomic functions can help predict cardiac outcomes and prevent possible adverse events in S1PR modulators treatment. Further studies with longer follow-ups are needed, especially for the second-generation of S1PR modulators, to confirm the safety profile of this therapeutic class.
Keywords: S1P receptor modulator; autonomic nervous system; cardiovascular effect; multiple sclerosis.
© The Author(s), 2022.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: V.C. has no conflict of interest. R.H. received travel grants by Celgene and Sanofi. K.A. received personal compensation from Biogen Idec, Sanofi, Alexion, Celgene, BMS, Merck and Roche for consulting service and speaker honoraria. T.Z. received personal compensation from Biogen, Bayer, BMS, Hexal, Merck, Novartis, Roche, Sanofi, Teva, and Viatris for consulting and speaking services, and additional financial support for research activities from Bayer, Biogen, Novartis, Teva, and Sanofi.
Figures
References
-
- Roy R, Alotaibi AA, Freedman MS. Sphingosine 1-phosphate receptor modulators for multiple sclerosis. CNS Drugs 2021; 35: 385–402. - PubMed
-
- Peyrin-Biroulet L, Christopher R, Behan D, et al.. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 2017; 16: 495–503. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

