Clinical predictors of treatment response to tiotropium add-on therapy in adult asthmatic patients: From multicenter real-world cohort data in Korea
- PMID: 36438190
- PMCID: PMC9679363
- DOI: 10.1016/j.waojou.2022.100720
Clinical predictors of treatment response to tiotropium add-on therapy in adult asthmatic patients: From multicenter real-world cohort data in Korea
Abstract
Background: Tiotropium, a long-acting muscarinic antagonist, is recommended for add-on therapy to inhaled corticosteroids (ICS)-long-acting beta 2 agonists (LABA) for severe asthma. However, real-world studies on the predictors of response to tiotropium are limited. We investigated the real-world use of tiotropium in asthmatic adult patients in Korea and we identified predictors of positive response to tiotropium add-on.
Methods: We performed a multicenter, retrospective, cohort study using data from the Cohort for Reality and Evolution of Adult Asthma in Korea (COREA). We enrolled asthmatic participants who took ICS-LABA with at least 2 consecutive lung function tests at 3-month intervals. We compared tiotropium users and non-users, as well as tiotropium responders and non-responders to predict positive responses to tiotropium, defined as 1) increase in forced expiratory volume in 1 s (FEV1) ≥ 10% or 100 mL; and 2) increase in asthma control test (ACT) score ≥3 after 3 months of treatment.
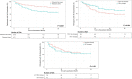
Results: The study included 413 tiotropium users and 1756 tiotropium non-users. Tiotropium users had low baseline lung function and high exacerbation rate, suggesting more severe asthma. Clinical predictors for positive response to tiotropium add-on were 1) positive bronchodilator response (BDR) [odds ratio (OR) = 6.8, 95% confidence interval (CI): 1.6-47.4, P = 0.021] for FEV1 responders; 2) doctor-diagnosed asthma-chronic obstructive pulmonary disease overlap (ACO) [OR = 12.6, 95% CI: 1.8-161.5, P = 0.024], and 3) initial ACT score <20 [OR = 24.1, 95% CI: 5.45-158.8, P < 0.001] for ACT responders. FEV1 responders also showed a longer exacerbation-free period than those with no FEV1 increase (P = 0.014), yielding a hazard ratio for the first asthma exacerbation of 0.5 (95% CI: 0.3-0.9, P = 0.016).
Conclusions: The results of this study suggest that tiotropium add-on for uncontrolled asthma with ICS-LABA would be more effective in patients with positive BDR or ACO. Additionally, an increase in FEV1 following tiotropium may predict a lower risk of asthma exacerbation.
Keywords: Asthma; Muscarinic antagonists; Predictor; Tiotropium; Treatment response.
© 2022 The Authors.
Figures
References
-
- Global strategy for asthma management and prevention. 2021. https://ginasthma.org
-
- Buendia J.A., Guerrero Patiño D., Cossio-Giraldo Y.E. Cost-effectiveness of tiotropium versus omalizumab for uncontrolled allergic asthma. J Asthma. 2021:1–8. - PubMed
-
- Hong S.H., Cho J.Y., Kim T.B., Lee E.K., Kwon S.H., Shin J.Y. Cost-effectiveness of tiotropium in elderly patients with severe asthma using real-world data. J Allergy Clin Immunol Pract. 2021;9:1939–1947. e7. - PubMed
LinkOut - more resources
Full Text Sources

