Transmission of SARS-CoV-2 during indoor clubbing events: A clustered randomized, controlled, multicentre trial protocol
- PMID: 36438274
- PMCID: PMC9687087
- DOI: 10.3389/fpubh.2022.981213
Transmission of SARS-CoV-2 during indoor clubbing events: A clustered randomized, controlled, multicentre trial protocol
Abstract
Introduction: The SARS-CoV-2 pandemic led to the implementation of several non-pharmaceutical interventions (NPIs), from closings of bars and restaurants to curfews and lockdowns. Vaccination campaigns started hoping it could efficiently alleviate NPI. The primary objective of the "Indoor Transmission of COVID-19" (ITOC) study is to determine among a fully vaccinated population the relative risk of SARS-CoV-2 transmission during one indoor clubbing event. Secondary objectives are to assess the transmission of other respiratory viruses, risk exposure, and attitudes toward COVID-19 vaccination, health pass, and psychological impact of indoor club closing.
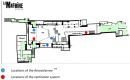
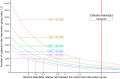
Methods and analysis: Four thousand four hundred healthy volunteers aged 18-49 years and fully vaccinated will be included in Paris region. The intervention is an 8-hour indoor clubbing event with no masks, no social distance, maximum room capacity, and ventilation. A reservation group of up to 10 people will recruit participants, who will be randomized 1:1 to either the experimental group (2,200 volunteers in two venues with capacities of 1,000 people each) or the control group (2,200 volunteers asked not to go to the club). All participants will provide a salivary sample on the day of the experiment and 7 days later. They also will answer several questionnaires. Virological analyses include polymerase chain reaction (PCR) of salivary samples and air of the venue, investigating SARS-CoV-2 and 18 respiratory viruses.
Ethics and dissemination: Ethical clearance was first obtained in France from the institutional review board (Comité de Protection des Personnes Ile de France VII - CPP), and the trial received clearance from the French National Agency for Medicines and Health Products (Agence National de Sécurité du Médicament - ANSM). The trial is supported and approved by The Agence Nationale Recherche sur le SIDA, les hépatites et maladies émergences (ANRS-MIE). Positive, negative, and inconclusive results will be published in peer-reviewed scientific journals.
Trial registration number: IDR-CB 2021-A01473-38. Clinicaltrial.gov, identifier: NCT05311865.
Keywords: SARS-CoV-2; nightclub; respiratory virus; respiratory virus transmission; vaccine.
Copyright © 2022 Goupil de Bouillé, Luong Nguyen, Crépey, Garlantezec, Doré, Dumas, Ben Mechlia, Tattevin, Gaudart, Spire, Lert, Yazdanpanah, Delaugerre, Noret and Zeggagh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

