mRNA-based vaccine technology for HIV
- PMID: 36438441
- PMCID: PMC9683993
- DOI: 10.15190/d.2022.9
mRNA-based vaccine technology for HIV
Abstract
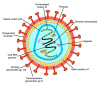
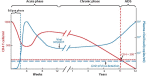
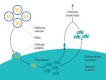
Human immunodeficiency virus (HIV) poses a major health problem around the globe, resulting in hundred-thousands of deaths from AIDS and over a million new infections annually. Although the standard treatment of HIV infection, antiretroviral therapy, has proven effective in preventing HIV transmission, it is unsuitable for worldwide use due to its substantial costs and frequent adverse effects. Besides promoting HIV/AIDS awareness through education, there is hardly an alternative for inhibiting the spread of the disease. One promising approach is the development of an HIV vaccine. Unfortunately, the high variability of envelope proteins from HIV subtypes, their frequency of mutation and the lack of fully understanding the mechanisms of protection against the virus constitute an obstacle for vaccine development. Efforts for developing successful anti-HIV vaccines have been underway for decades now, with little success. Lately, significant progress has been made in adopting the novel mRNA vaccine approach as an anti-HIV strategy. mRNA vaccines received a great thrust during the COVID-19 pandemic. Now, several mRNA-based HIV vaccines are undergoing clinical trials to evaluate their safety and efficacy. This review offers an overview of the pathogenesis and treatment of HIV / AIDS, previous efforts of HIV vaccine development and introduces mRNA vaccines as a promising and potential game changing platform for HIV vaccination.
Keywords: AIDS; HIV; antiretroviral therapy; bNAbs; mRNA.; vaccine.
Copyright © 2022, Fortner A et al., Applied Systems and Discoveries Journals.
Conflict of interest statement
Conflict of interests: The authors declare no conflicts of interest.
Figures


References
-
- Roser Max, Ritchie Hannah HIV / AIDS. . Our World in Data. 2018. https://ourworldindata.org/hiv-aids https://ourworldindata.org/hiv-aids
-
- HIV. World Health Organization; Accessed on March 30, 2022. 2022. https://www.who.int/news-room/fact-sheets/detail/hiv-aids https://www.who.int/news-room/fact-sheets/detail/hiv-aids
-
- U.S. Statistics. HIV.gov; Accessed on March 30, 2022. 2022. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
Publication types
LinkOut - more resources
Full Text Sources
