Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss
- PMID: 36438479
- PMCID: PMC9691373
- DOI: 10.7150/thno.78467
Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss
Abstract
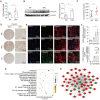
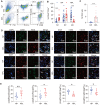
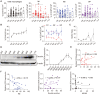
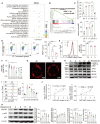
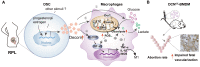
Rationale: Recurrent pregnancy loss (RPL) is a distressing disorder that seriously affects the physical and psychological health of women. RPL is also a sentinel risk marker for future obstetric complications and warrants in-depth investigation. Abnormal polarization and functions of decidual macrophages are associated with RPL; however, the underlying mechanisms remain poorly understood. Methods: Decorin expression, localization, and content in the decidua of women with normal pregnancy (NP) and those with RPL were measured using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), western blotting, immunofluorescence, and enzyme-linked immunosorbent assay. The profiles of decidual macrophage subsets were determined using flow cytometry and immunofluorescence in both groups. The correlation between decorin content and the proportion of decidual macrophage subsets in the decidua of early NP women was determined using Pearson analysis. The effects of decorin on the polarization and functions of macrophages were assessed in an in-vitro model of Raw264.7 cells via flow cytometry, western blotting, and RT-qPCR. Moreover, the mitochondrial metabolism in Raw264.7 cells under decorin administration was measured via flow cytometry, western blotting, and immunofluorescence. Thirty-three pregnant mice were included in the in vivo model and underwent different treatments. The embryo abortion rate, macrophage phenotype in the spleen and uteri, and placental development were evaluated using flow cytometry and hematoxylin-eosin staining. Results: Decorin, derived from decidual stromal cells, was highly expressed in the decidua of women with RPL. A positive correlation between decorin content and the proportion of M1-like macrophages was also observed in the decidua of early NP women. In vitro studies showed that decorin treatment inhibited macrophage polarization to M2-like subsets and boosted the inflammatory response, which was related to enhanced anaerobic glycolysis, increased mitochondrial membrane potential and intracellular reactive oxygen species levels, reduced mitochondrial mass, and activation of the myeloid differentiation primary response 88-nuclear factor-κB signaling pathway. Adoptive transfer of decorin-treated bone marrow-derived macrophages in pregnant C57BL/6 mice increased the embryo absorption, accompanied by impaired fetal vascularization. Conclusions: Decidual stromal cell-derived decorin can polarize decidual macrophages toward the M1 phenotype by regulating mitochondrial metabolism, resulting in the occurrence of RPL.
Keywords: decidual macrophages; decidual stromal cells; decorin; mitochondrial metabolism; normal pregnancy; recurrent pregnancy loss.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6(1):98. - PubMed
-
- Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. - PubMed
-
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658–67. - PubMed
-
- Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, Al-Memar M, Brewin J, Abrahams VM. et al. Recurrent miscarriage: evidence to accelerate action. Lancet. 2021;397:1675–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

