Choroidal layer segmentation in OCT images by a boundary enhancement network
- PMID: 36438560
- PMCID: PMC9691264
- DOI: 10.3389/fcell.2022.1060241
Choroidal layer segmentation in OCT images by a boundary enhancement network
Abstract
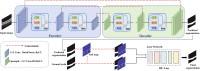
Morphological changes of the choroid have been proved to be associated with the occurrence and pathological mechanism of many ophthalmic diseases. Optical Coherence Tomography (OCT) is a non-invasive technique for imaging of ocular biological tissues, that can reveal the structure of the retinal and choroidal layers in micron-scale resolution. However, unlike the retinal layer, the interface between the choroidal layer and the sclera is ambiguous in OCT, which makes it difficult for ophthalmologists to identify with certainty. In this paper, we propose a novel boundary-enhanced encoder-decoder architecture for choroid segmentation in retinal OCT images, in which a Boundary Enhancement Module (BEM) forms the backbone of each encoder-decoder layer. The BEM consists of three parallel branches: 1) a Feature Extraction Branch (FEB) to obtain feature maps with different receptive fields; 2) a Channel Enhancement Branch (CEB) to extract the boundary information of different channels; and 3) a Boundary Activation Branch (BAB) to enhance the boundary information via a novel activation function. In addition, in order to incorporate expert knowledge into the segmentation network, soft key point maps are generated on the choroidal boundary, and are combined with the predicted images to facilitate precise choroidal boundary segmentation. In order to validate the effectiveness and superiority of the proposed method, both qualitative and quantitative evaluations are employed on three retinal OCT datasets for choroid segmentation. The experimental results demonstrate that the proposed method yields better choroid segmentation performance than other deep learning approaches. Moreover, both 2D and 3D features are extracted for statistical analysis from normal and highly myopic subjects based on the choroid segmentation results, which is helpful in revealing the pathology of high myopia. Code is available at https://github.com/iMED-Lab/Choroid-segmentation.
Keywords: boundary segmentation; choroidal layer; deep learning; high myopia; optical coherence tomography.
Copyright © 2022 Wu, Gong, Hao, Zhang, Su, Yan, Ma and Zhao.
Figures










References
-
- Chai Z., Zhou K., Yang J., Ma Y., Chen Z., Gao S., et al. (2020). “Perceptual-assisted adversarial adaptation for choroid segmentation in optical coherence tomography,” in 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), Iowa City, IA, United States, April 3–7, 2020 (IEEE; ), 1966.
LinkOut - more resources
Full Text Sources
Miscellaneous

