Non-muscle myosin II and the plasticity of 3D cell migration
- PMID: 36438570
- PMCID: PMC9691290
- DOI: 10.3389/fcell.2022.1047256
Non-muscle myosin II and the plasticity of 3D cell migration
Abstract
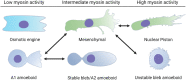
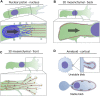
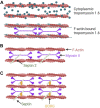
Confined cells migrating through 3D environments are also constrained by the laws of physics, meaning for every action there must be an equal and opposite reaction for cells to achieve motion. Fascinatingly, there are several distinct molecular mechanisms that cells can use to move, and this is reflected in the diverse ways non-muscle myosin II (NMII) can generate the mechanical forces necessary to sustain 3D cell migration. This review summarizes the unique modes of 3D migration, as well as how NMII activity is regulated and localized within each of these different modes. In addition, we highlight tropomyosins and septins as two protein families that likely have more secrets to reveal about how NMII activity is governed during 3D cell migration. Together, this information suggests that investigating the mechanisms controlling NMII activity will be helpful in understanding how a single cell transitions between distinct modes of 3D migration in response to the physical environment.
Keywords: cytoskeleton; matrix; mechanotranduction; motility; myosin.
Copyright © 2022 Cowan, Duggan, Hewitt and Petrie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anderson T. W., Vaughan A. N., Cramer L. P. (2008). Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol. Biol. Cell 19, 5006–5018. 10.1091/mbc.e08-01-0034 - DOI - PMC - PubMed
-
- Appaduray M. A., Masedunskas A., Bryce N. S., Lucas C. A., Warren S. C., Timpson P., et al. (2016). Recruitment kinetics of tropomyosin Tpm3.1 to actin filament bundles in the cytoskeleton is independent of actin filament kinetics. PLoS One 11, e0168203. 10.1371/journal.pone.0168203 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

