Endothelial cilia dysfunction in pathogenesis of hereditary hemorrhagic telangiectasia
- PMID: 36438574
- PMCID: PMC9686338
- DOI: 10.3389/fcell.2022.1037453
Endothelial cilia dysfunction in pathogenesis of hereditary hemorrhagic telangiectasia
Abstract
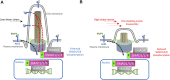
Hereditary hemorrhagic telangiectasia (HHT) is associated with defective capillary network, leading to dilated superficial vessels and arteriovenous malformations (AVMs) in which arteries connect directly to the veins. Loss or haploinsufficiency of components of TGF-β signaling, ALK1, ENG, SMAD4, and BMP9, have been implicated in the pathogenesis AVMs. Emerging evidence suggests that the inability of endothelial cells to detect, transduce and respond to blood flow, during early development, is an underpinning of AVM pathogenesis. Therefore, components of endothelial flow detection may be instrumental in potentiating TGF-β signaling in perfused blood vessels. Here, we argue that endothelial cilium, a microtubule-based and flow-sensitive organelle, serves as a signaling hub by coupling early flow detection with potentiation of the canonical TGF-β signaling in nascent endothelial cells. Emerging evidence from animal models suggest a role for primary cilia in mediating vascular development. We reason, on recent observations, that endothelial cilia are crucial for vascular development and that embryonic loss of endothelial cilia will curtail TGF-β signaling, leading to associated defects in arteriovenous development and impaired vascular stability. Loss or dysfunction of endothelial primary cilia may be implicated in the genesis of AVMs due, in part, to inhibition of ALK1/SMAD4 signaling. We speculate that AVMs constitute part of the increasing spectrum of ciliopathy-associated vascular defects.
Keywords: BMP signaling; Endothelial cilia; TGF-β; Vascular disease; Zebrafish.
Copyright © 2022 Eisa-Beygi, Burrows and Link.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Brain arteriovenous malformation in hereditary hemorrhagic telangiectasia: Recent advances in cellular and molecular mechanisms.Front Hum Neurosci. 2022 Nov 24;16:1006115. doi: 10.3389/fnhum.2022.1006115. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36504622 Free PMC article. Review.
-
SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2.Circulation. 2018 Nov 20;138(21):2379-2394. doi: 10.1161/CIRCULATIONAHA.118.033842. Circulation. 2018. PMID: 29976569 Free PMC article.
-
Angiopoietin-2 Inhibition Rescues Arteriovenous Malformation in a Smad4 Hereditary Hemorrhagic Telangiectasia Mouse Model.Circulation. 2019 Apr 23;139(17):2049-2063. doi: 10.1161/CIRCULATIONAHA.118.036952. Circulation. 2019. PMID: 30744395 Free PMC article.
-
Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of Hereditary Hemorrhagic Telangiectasia.Angiogenesis. 2018 May;21(2):363-380. doi: 10.1007/s10456-018-9602-0. Epub 2018 Feb 19. Angiogenesis. 2018. PMID: 29460088 Free PMC article.
-
Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia.Front Genet. 2015 Feb 11;6:35. doi: 10.3389/fgene.2015.00035. eCollection 2015. Front Genet. 2015. PMID: 25717337 Free PMC article. Review.
Cited by
-
Human iPSCs as Model Systems for BMP-Related Rare Diseases.Cells. 2023 Sep 2;12(17):2200. doi: 10.3390/cells12172200. Cells. 2023. PMID: 37681932 Free PMC article. Review.
References
-
- Akla N., Viallard C., Popovic N., Lora Gil C., Sapieha P., Larrivée B. (2018). BMP9 (bone morphogenetic protein-9)/alk1 (Activin-Like kinase receptor type I) signaling prevents hyperglycemia-induced vascular permeability. Arterioscler. Thromb. Vasc. Biol. 38, 1821–1836. 10.1161/ATVBAHA.118.310733 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

