Neo-Adjuvant Use of Sorafenib for Hepatocellular Carcinoma Awaiting Liver Transplantation
- PMID: 36438781
- PMCID: PMC9681796
- DOI: 10.3389/ti.2022.10569
Neo-Adjuvant Use of Sorafenib for Hepatocellular Carcinoma Awaiting Liver Transplantation
Abstract
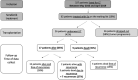
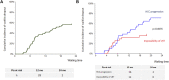
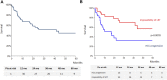
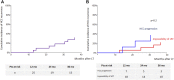
Data on efficacy and safety of sorafenib in a neoadjuvant setting for HCC awaiting liver transplantation (LT) are heterogeneous and scarce. We aimed to investigate the trajectory of patients treated with sorafenib while awaiting LT. All patients listed for HCC and treated with sorafenib were included in a monocentric observational study. A clinical and biological evaluation was performed every month. Radiological tumor response evaluation was realized every 3 months on the waiting list and every 6 months after LT. Among 327 patients listed for HCC, 62 (19%) were treated with Sorafenib. Sorafenib was initiated for HCC progression after loco-regional therapy (LRT) in 50% of cases and for impossibility of LRT in 50% of cases. The mean duration of treatment was 6 months. Thirty six patients (58%) dropped-out for tumor progression and 26 (42%) patients were transplanted. The 5-year overall and recurrent-free survival after LT was 77% and 48% respectively. Patients treated for impossibility of LRT had acceptable 5-year intention-to-treat overall and post-LT survivals. Conversely, patients treated for HCC progression presented high dropout rate and low intention-to-treat survival. Our results suggest that it is very questionable in terms of utility that patients treated for HCC progression should even be kept listed once the tumor progression has been observed.
Keywords: cancer; cancer-therapeutics; hepatocellular carcinoma (HCC); liver transplantation; neoadjuvant therapy; sorafenib; tyrosine kinase inhibitor.
Copyright © 2022 Minoux, Lassailly, Ningarhari, Lubret, El Amrani, Canva, Truant, Mathurin, Louvet, Lebuffe, Goria, Nguyen-Khac, Boleslawski and Dharancy.
Conflict of interest statement
SD reports receiving lecture fees from Novartis, Sandroz, Intercept, Astellas, Roche, Ipsen and Abbvie and serving as a board member of Novartis and Biotest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agopian VG, Morshedi MM, McWilliams J, Harlander-Locke MP, Markovic D, Zarrinpar A, et al. Complete Pathologic Response to Pretransplant Locoregional Therapy for Hepatocellular Carcinoma Defines Cancer Cure after Liver Transplantation: Analysis of 501 Consecutively Treated Patients. Ann Surg (2015) 262(3):536–45. 10.1097/SLA.0000000000001384 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

