Human periventricular nodular heterotopia shows several interictal epileptic patterns and hyperexcitability of neuronal firing
- PMID: 36438938
- PMCID: PMC9695411
- DOI: 10.3389/fneur.2022.1022768
Human periventricular nodular heterotopia shows several interictal epileptic patterns and hyperexcitability of neuronal firing
Abstract
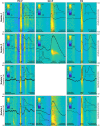
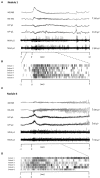
Periventricular nodular heterotopia (PNH) is a malformation of cortical development that frequently causes drug-resistant epilepsy. The epileptogenicity of ectopic neurons in PNH as well as their role in generating interictal and ictal activity is still a matter of debate. We report the first in vivo microelectrode recording of heterotopic neurons in humans. Highly consistent interictal patterns (IPs) were identified within the nodules: (1) Periodic Discharges PLUS Fast activity (PD+F), (2) Sporadic discharges PLUS Fast activity (SD+F), and (3) epileptic spikes (ES). Neuronal firing rates were significantly modulated during all IPs, suggesting that multiple IPs were generated by the same local neuronal populations. Furthermore, firing rates closely followed IP morphologies. Among the different IPs, the SD+F pattern was found only in the three nodules that were actively involved in seizure generation but was never observed in the nodule that did not take part in ictal discharges. On the contrary, PD+F and ES were identified in all nodules. Units that were modulated during the IPs were also found to participate in seizures, increasing their firing rate at seizure onset and maintaining an elevated rate during the seizures. Together, nodules in PNH are highly epileptogenic and show several IPs that provide promising pathognomonic signatures of PNH. Furthermore, our results show that PNH nodules may well initiate seizures.
Keywords: epilepsy; human; in vivo; interictal; microelectrode; periventricular nodular heterotopia; seizure.
Copyright © 2022 Frazzini, Whitmarsh, Lehongre, Yger, Lemarechal, Mathon, Adam, Hasboun, Lambrecq and Navarro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Battaglia G, Franceschetti S, Chiapparini L, Freri E, Bassanini S, Giavazzi A, et al. . Electroencephalographic recordings of focal seizures in patients affected by periventricular nodular heterotopia: role of the heterotopic nodules in the genesis of epileptic discharges. J Child Neurol. (2005) 20:369–77. 10.1177/08830738050200041701 - DOI - PubMed
LinkOut - more resources
Full Text Sources

