Plasma biomarkers of vascular dysfunction uniquely relate to a vascular-risk profile of neurocognitive deficits in virally-suppressed adults with HIV
- PMID: 36439059
- PMCID: PMC9685288
- DOI: 10.1016/j.bbih.2022.100560
Plasma biomarkers of vascular dysfunction uniquely relate to a vascular-risk profile of neurocognitive deficits in virally-suppressed adults with HIV
Abstract
Objective: Chronic inflammation and vascular dysfunction (e.g., chronic endothelial activation) are related yet dissociable mechanisms of HIV-associated neurocognitive impairment (NCI), even among those on antiretroviral therapy (ART). However, how these mechanisms differentially contribute to domain-specific deficits in people with and without HIV (PWH, PWoH) is unclear. We empirically-derived profiles of NCI and examined relationships with peripheral inflammatory and vascular biomarkers.
Methods: Participants were 84 virally-suppressed PWH and 126 PWoH who underwent neuropsychological testing and blood draw. Cluster analysis identified subgroups based on domain deficit scores. ANOVAs examined HIV serostatus and cluster group differences in composite plasma biomarker z-scores of inflammation (IL-6, CXCL10/IP-10, CCL2/MCP-1) and vascular injury (VCAM-1, ICAM-1, uPAR). Confirmatory regressions examined the interaction of HIV and biomarker z-scores on domain-specific T-scores, controlling for cardiovascular disease (CVD) risk and psychosocial factors.
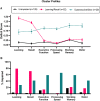
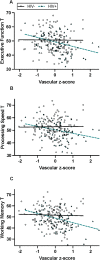
Results: Cluster analysis identified three groups: Unimpaired (n = 129), Learning/Recall (n = 52, isolated learning/recall deficits), Dysexecutive/Slow (n = 29, executive function, working memory, processing speed, and motor deficits). PWH had higher odds of Dysexecutive/Slow membership, which related to CVD risk and higher vascular dysfunction, but not inflammation, in PWH. Vascular biomarkers moderated adverse HIV effects on executive function, processing speed, and working memory such that PWH had lower T-scores only when vascular dysfunction was high.
Conclusions: In PWH with controlled disease, peripheral markers of endothelial dysfunction and vascular permeability are selectively associated with an empirically-derived subgroup that exhibits domain deficits commonly impacted by cerebrovascular disease. Findings support the presence of a vascular NCI subgroup of PWH who may benefit from interventions that directly target the neurovascular unit.
Keywords: Cluster analysis; Endothelial dysfunction; Executive function; HIV-Associated neurocognitive disorder; IP-10; Inflammation; MCP-1; Processing speed; VCAM-1; Vascular dementia.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Identifying and distinguishing cognitive profiles among virally suppressed people with HIV.Neuropsychology. 2024 Feb;38(2):169-183. doi: 10.1037/neu0000935. Epub 2023 Nov 16. Neuropsychology. 2024. PMID: 37971860 Free PMC article.
-
Vascular injury markers associated with cognitive impairment in people with HIV on suppressive antiretroviral therapy.AIDS. 2023 Nov 15;37(14):2137-2147. doi: 10.1097/QAD.0000000000003675. Epub 2023 Jul 27. AIDS. 2023. PMID: 37503603 Free PMC article.
-
Plasma Inflammatory Biomarkers Link to Worse Cognition Among Africans with HIV.J Acquir Immune Defic Syndr. 2025 Apr 8. doi: 10.1097/QAI.0000000000003679. Online ahead of print. J Acquir Immune Defic Syndr. 2025. PMID: 40199254
-
Aging with HIV and HIV-associated neurocognitive impairment.AIDS. 2025 Mar 1;39(3):215-228. doi: 10.1097/QAD.0000000000004057. Epub 2025 Jan 30. AIDS. 2025. PMID: 39878669 Review.
-
A Meta-Analytic Review of the Effect of Antiretroviral Therapy on Neurocognitive Outcomes in Adults Living with HIV-1 in Low-and Middle-Income Countries.Neuropsychol Rev. 2022 Dec;32(4):828-854. doi: 10.1007/s11065-021-09527-y. Epub 2021 Nov 10. Neuropsychol Rev. 2022. PMID: 34757490 Review.
Cited by
-
Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review.Life (Basel). 2024 Apr 15;14(4):508. doi: 10.3390/life14040508. Life (Basel). 2024. PMID: 38672778 Free PMC article. Review.
-
HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology.Int J Mol Sci. 2024 Apr 25;25(9):4697. doi: 10.3390/ijms25094697. Int J Mol Sci. 2024. PMID: 38731913 Free PMC article. Review.
-
Circulating Plasma Exosomal Proteins of Either SHIV-Infected Rhesus Macaque or HIV-Infected Patient Indicates a Link to Neuropathogenesis.Viruses. 2023 Mar 21;15(3):794. doi: 10.3390/v15030794. Viruses. 2023. PMID: 36992502 Free PMC article.
-
Lack of Association of Vascular Risk Factors with HIV-Associated Neurocognitive Disorders in cART-Treated Adults Aged ≥ 50 Years in Tanzania.Viruses. 2024 May 22;16(6):819. doi: 10.3390/v16060819. Viruses. 2024. PMID: 38932112 Free PMC article.
-
HIV-1 RNA in extracellular vesicles is associated with neurocognitive outcomes.Nat Commun. 2024 May 23;15(1):4391. doi: 10.1038/s41467-024-48644-z. Nat Commun. 2024. PMID: 38782925 Free PMC article.
References
-
- Anderson A.M., Jang J.H., Easley K.A., Fuchs D., Gisslen M., Zetterberg H., Blennow K., Ellis R.J., Franklin D., Heaton R.K., Grant I., Letendre S.L. Cognitive and neuronal link with inflammation: a longitudinal study in people with and without HIV infection. J. Acquir. Immune Defic. Syndr. 2020;85:617–625. 1999. - PMC - PubMed
-
- Anderson A.M., Muñoz-Moreno J.A., McClernon D., Ellis R.J., Cookson D., Clifford D.B., Collier A.C., Gelman B.B., Marra C.M., McArthur J.C. Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J. Infect. Dis. 2016;215(1):105–113. jiw505. - PMC - PubMed
-
- Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M., Clifford D.B., Cinque P., Epstein L.G., Goodkin K., Gisslen M., Grant I., Heaton R.K., Joseph J., Marder K., Marra C.M., McArthur J.C., Nunn M., Price R.W., Pulliam L., Robertson K.R., Sacktor N., Valcour V., Wojna V.E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

