Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration
- PMID: 36439087
- PMCID: PMC9676151
- DOI: 10.1016/j.bioactmat.2022.10.019
Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration
Erratum in
-
Corrigendum to "Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration" [Bioact. Mater. 23 (2023) 247-260].Bioact Mater. 2022 Dec 20;24:171-173. doi: 10.1016/j.bioactmat.2022.12.008. eCollection 2023 Jun. Bioact Mater. 2022. PMID: 36606256 Free PMC article.
Abstract
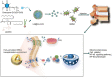
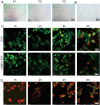
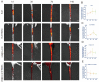
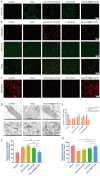
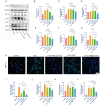
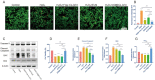
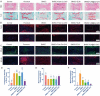
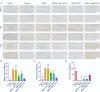
Stem cell transplantation has been proved a promising therapeutic instrument in intervertebral disc degeneration (IVDD). However, the elevation of oxidative stress in the degenerated region impairs the efficiency of mesenchymal stem cells (BMSCs) transplantation treatment via exaggeration of mitochondrial ROS and promotion of BMSCs apoptosis. Herein, we applied an emulsion-confined assembly method to encapsulate Coenzyme Q10 (Co-Q10), a promising hydrophobic antioxidant which targets mitochondria ROS, into the lecithin micelles, which renders the insoluble Co-Q10 dispersible in water as stable colloids. These micelles are injectable, which displayed efficient ability to facilitate Co-Q10 to get into BMSCs in vitro, and exhibited prolonged release of Co-Q10 in intervertebral disc tissue of animal models. Compared to mere use of Co-Q10, the Co-Q10 loaded micelle possessed better bioactivities, which elevated the viability, restored mitochondrial structure as well as function, and enhanced production of ECM components in rat BMSCs. Moreover, it is demonstrated that the injection of this micelle with BMSCs retained disc height and alleviated IVDD in a rat needle puncture model. Therefore, these Co-Q10 loaded micelles play a protective role in cell survival and differentiation through antagonizing mitochondrial ROS, and might be a potential therapeutic agent for IVDD.
Keywords: Coenzyme Q10; Intervertebral disc degeneration; Mesenchymal stem cell; Micelle; Reactive oxygen species.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Stevans J.M., Delitto A., Khoja S.S., Patterson C.G., Smith C.N., Schneider M.J., Freburger J.K., Greco C.M., Freel J.A., Sowa G.A., Wasan A.D., Brennan G.P., Hunter S.J., Minick K.I., Wegener S.T., Ephraim P.L., Friedman M., Beneciuk J.M., George S.Z., Saper R.B. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37371. - DOI - PMC - PubMed
-
- Li J., Marmorat C., Vasilyev G., Jiang J., Koifman N., Guo Y., Talmon I., Zussman E., Gersappe D., Davis R., Rafailovich M. Flow induced stability of pluronic hydrogels: injectable and unencapsulated nucleus pulposus replacement. Acta Biomater. 2019;96:295–302. doi: 10.1016/j.actbio.2019.07.021. - DOI - PubMed
LinkOut - more resources
Full Text Sources

