Dorsal switch protein 1 as a damage signal in insect gut immunity to activate dual oxidase via an eicosanoid, PGE2
- PMID: 36439105
- PMCID: PMC9691268
- DOI: 10.3389/fimmu.2022.994626
Dorsal switch protein 1 as a damage signal in insect gut immunity to activate dual oxidase via an eicosanoid, PGE2
Abstract
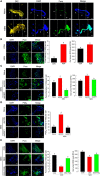
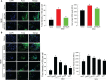
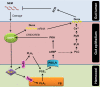
Various microbiota including beneficial symbionts reside in the insect gut. Infections of pathogens cause dysregulation of the microflora and threaten insect survival. Reactive oxygen species (ROS) have been used in the gut immune responses, in which its production is tightly regulated by controlling dual oxidase (Duox) activity via Ca2+ signal to protect beneficial microflora and gut epithelium due to its high cytotoxicity. However, it was not clear how the insects discriminate the pathogens from the various microbes in the gut lumen to trigger ROS production. An entomopathogenic nematode (Steinernema feltiae) infection elevated ROS level in the gut lumen of a lepidopteran insect, Spodoptera exigua. Dorsal switch protein 1 (DSP1) localized in the nucleus in the midgut epithelium was released into plasma upon the nematode infection and activated phospholipase A2 (PLA2). The activated PLA2 led to an increase of PGE2 level in the midgut epithelium, in which rising Ca2+ signal up-regulated ROS production. Inhibiting DSP1 release by its specific RNA interference (RNAi) or specific inhibitor, 3-ethoxy-4-methoxyphenol, treatment failed to increase the intracellular Ca2+ level and subsequently prevented ROS production upon the nematode infection. A specific PLA2 inhibitor treatment also prevented the up-regulation of Ca2+ and subsequent ROS production upon the nematode infection. However, the addition of PGE2 to the inhibitor treatment rescued the gut immunity. DSP1 release was not observed at infection with non-pathogenic pathogens but detected in plasma with pathogenic infections that would lead to damage to the gut epithelium. These results indicate that DSP1 acts as a damage-associated molecular pattern in gut immunity through DSP1/PLA2/Ca2+/Duox.
Keywords: DSP1; PGE2; eicosanoid; gut; immunity; insect.
Copyright © 2022 Roy, Ahmed and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
HMGB1-Like Dorsal Switch Protein 1 Triggers a Damage Signal in Mosquito Gut to Activate Dual Oxidase via Eicosanoids.J Innate Immun. 2022;14(6):657-672. doi: 10.1159/000524561. Epub 2022 May 5. J Innate Immun. 2022. PMID: 35512659 Free PMC article.
-
Damage signal induced by Bacillus thuringiensis infection triggers immune responses via a DAMP molecule in lepidopteran insect, Spodoptera exigua.Dev Comp Immunol. 2023 Feb;139:104559. doi: 10.1016/j.dci.2022.104559. Epub 2022 Sep 29. Dev Comp Immunol. 2023. PMID: 36181778
-
PGE2 upregulates gene expression of dual oxidase in a lepidopteran insect midgut via cAMP signalling pathway.Open Biol. 2020 Oct;10(10):200197. doi: 10.1098/rsob.200197. Epub 2020 Oct 21. Open Biol. 2020. PMID: 33081632 Free PMC article.
-
Regulatory mechanisms of microbial homeostasis in insect gut.Insect Sci. 2021 Apr;28(2):286-301. doi: 10.1111/1744-7917.12868. Epub 2020 Sep 23. Insect Sci. 2021. PMID: 32888254 Review.
-
Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues.Genes (Basel). 2021 Feb 1;12(2):211. doi: 10.3390/genes12020211. Genes (Basel). 2021. PMID: 33535438 Free PMC article. Review.
Cited by
-
Alveolar macrophage-derived gVPLA2 promotes ventilator-induced lung injury via the cPLA2/PGE2 pathway.BMC Pulm Med. 2023 Dec 6;23(1):494. doi: 10.1186/s12890-023-02793-x. BMC Pulm Med. 2023. PMID: 38057837 Free PMC article.
-
Immune functions of pattern recognition receptors in Lepidoptera.Front Immunol. 2023 Jun 16;14:1203061. doi: 10.3389/fimmu.2023.1203061. eCollection 2023. Front Immunol. 2023. PMID: 37398667 Free PMC article. Review.
-
Metabolic changes and potential biomarkers in "Candidatus Liberibacter solanacearum"-infected potato psyllids: implications for psyllid-pathogen interactions.Front Plant Sci. 2023 Jul 19;14:1204305. doi: 10.3389/fpls.2023.1204305. eCollection 2023. Front Plant Sci. 2023. PMID: 37538064 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

