Generation of high affinity ICAM-1-specific nanobodies and evaluation of their suitability for allergy treatment
- PMID: 36439110
- PMCID: PMC9682242
- DOI: 10.3389/fimmu.2022.1022418
Generation of high affinity ICAM-1-specific nanobodies and evaluation of their suitability for allergy treatment
Abstract
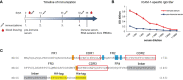
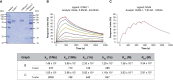
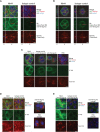
The nasal cavity is an important site of allergen entry. Hence, it represents an organ where trans-epithelial allergen penetration and subsequent IgE-mediated allergic inflammation can potentially be inhibited. Intercellular adhesion molecule 1 (ICAM-1) is highly expressed on the surface of respiratory epithelial cells in allergic patients. It was identified as a promising target to immobilize antibody conjugates bispecific for ICAM-1 and allergens and thereby block allergen entry. We have previously characterized a nanobody specific for the major birch pollen allergen Bet v 1 and here we report the generation and characterization of ICAM-1-specific nanobodies. Nanobodies were obtained from a camel immunized with ICAM-1 and a high affinity binder was selected after phage display (Nb44). Nb44 was expressed as recombinant protein containing HA- and His-tags in Escherichia coli (E.coli) and purified via affinity chromatography. SDS-PAGE and Western blot revealed a single band at approximately 20 kDa. Nb44 bound to recombinant ICAM-1 in ELISA, and to ICAM-1 expressed on the human bronchial epithelial cell line 16HBE14o- as determined by flow cytometry. Experiments conducted at 4°C and at 37°C, to mimic physiological conditions, yielded similar percentages (97.2 ± 1.2% and 96.7 ± 1.5% out of total live cells). To confirm and visualize binding, we performed immunofluorescence microscopy. While Texas Red Dextran was rapidly internalized Nb44 remained localized on the cell surface. Additionally, we determined the strength of Nb44 and ICAM-1 interaction using surface plasmon resonance (SPR). Nb44 bound ICAM-1 with high affinity (10-10 M) and had slow off-rates (10-4 s-1). In conclusion, our results showed that the selected ICAM-1-specific nanobody bound ICAM-1 with high affinity and was not internalized. Thus, it could be further used to engineer heterodimers with allergen-specific nanobodies in order to develop topical treatments of pollen allergy.
Keywords: ICAM-1; VHH; allergy; high affinity; nanobody.
Copyright © 2022 Zettl, Ivanova, Zghaebi, Rutovskaya, Ellinger, Goryainova, Kollárová, Villazala-Merino, Lupinek, Weichwald, Drescher, Eckl-Dorna, Tillib and Flicker.
Conflict of interest statement
AD is employed by Cytiva GmbH. CL reports personal fees from Thermofisher, outside the submitted work. JE-D reports grants and personal fees from Astrazeneca, personal fees from Sanofi and GSK, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Figures


Similar articles
-
Isolation of nanobodies with potential to reduce patients' IgE binding to Bet v 1.Allergy. 2022 Jun;77(6):1751-1760. doi: 10.1111/all.15191. Epub 2021 Dec 16. Allergy. 2022. PMID: 34837242
-
Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection.Int J Mol Sci. 2023 Feb 1;24(3):2725. doi: 10.3390/ijms24032725. Int J Mol Sci. 2023. PMID: 36769047 Free PMC article.
-
Production and Characterization of Novel Camel Single Domain Antibody Targeting Mouse Vascular Endothelial Growth Factor.Monoclon Antib Immunodiagn Immunother. 2016 Jun;35(3):167-71. doi: 10.1089/mab.2016.0001. Epub 2016 May 11. Monoclon Antib Immunodiagn Immunother. 2016. PMID: 27167350
-
Nanobodies-Useful Tools for Allergy Treatment?Front Immunol. 2020 Sep 30;11:576255. doi: 10.3389/fimmu.2020.576255. eCollection 2020. Front Immunol. 2020. PMID: 33117377 Free PMC article. Review.
-
[Rhinitis in adults].Acta Med Croatica. 2011;65(2):181-7. Acta Med Croatica. 2011. PMID: 22359885 Review. Croatian.
Cited by
-
Appraisal terpenoids rich Boswellia carterri ethyl acetate extract in binary cyclodextrin oligomer nano complex for improving respiratory distress.Sci Rep. 2024 Jul 22;14(1):16779. doi: 10.1038/s41598-024-66297-2. Sci Rep. 2024. PMID: 39039094 Free PMC article.
-
Extract-Shaped Immune Repertoires as Source for Nanobody-Based Human IgE in Grass Pollen Allergy.Mol Biotechnol. 2023 Sep;65(9):1518-1527. doi: 10.1007/s12033-023-00664-8. Epub 2023 Jan 25. Mol Biotechnol. 2023. PMID: 36696011
-
Single-Domain Antibodies-Novel Tools to Study and Treat Allergies.Int J Mol Sci. 2024 Jul 11;25(14):7602. doi: 10.3390/ijms25147602. Int J Mol Sci. 2024. PMID: 39062843 Free PMC article. Review.
-
Trimeric Bet v 1-specific nanobodies cause strong suppression of IgE binding.Front Immunol. 2024 May 3;15:1343024. doi: 10.3389/fimmu.2024.1343024. eCollection 2024. Front Immunol. 2024. PMID: 38784378 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

