Development and evaluation of low-volume tests to detect and characterize antibodies to SARS-CoV-2
- PMID: 36439154
- PMCID: PMC9682908
- DOI: 10.3389/fimmu.2022.968317
Development and evaluation of low-volume tests to detect and characterize antibodies to SARS-CoV-2
Abstract
Low-volume antibody assays can be used to track SARS-CoV-2 infection rates in settings where active testing for virus is limited and remote sampling is optimal. We developed 12 ELISAs detecting total or antibody isotypes to SARS-CoV-2 nucleocapsid, spike protein or its receptor binding domain (RBD), 3 anti-RBD isotype specific luciferase immunoprecipitation system (LIPS) assays and a novel Spike-RBD bridging LIPS total-antibody assay. We utilized pre-pandemic (n=984) and confirmed/suspected recent COVID-19 sera taken pre-vaccination rollout in 2020 (n=269). Assays measuring total antibody discriminated best between pre-pandemic and COVID-19 sera and were selected for diagnostic evaluation. In the blind evaluation, two of these assays (Spike Pan ELISA and Spike-RBD Bridging LIPS assay) demonstrated >97% specificity and >92% sensitivity for samples from COVID-19 patients taken >21 days post symptom onset or PCR test. These assays offered better sensitivity for the detection of COVID-19 cases than a commercial assay which requires 100-fold larger serum volumes. This study demonstrates that low-volume in-house antibody assays can provide good diagnostic performance, and highlights the importance of using well-characterized samples and controls for all stages of assay development and evaluation. These cost-effective assays may be particularly useful for seroprevalence studies in low and middle-income countries.
Keywords: COVID-19; ELISA; SARS-CoV-2; antibody; diagnostic; evaluation; immunity; luciferase immunoprecipitation system (LIPS).
Copyright © 2022 Halliday, Long, Baum, Thomas, Shelley, Oliver, Gupta, Francis, Williamson, Di Bartolo, Randell, Ben-Khoud, Kelland, Mortimer, Ball, Plumptre, Chandler, Obst, Secchi, Piemonti, Lampasona, Smith, Gregorova, Knezevic, Metz, Barr, Morales-Aza, Oliver, Collingwood, Hitchings, Ring, Wooldridge, Rivino, Timpson, McKernon, Muir, Hamilton, Arnold, Woolfson, Goenka, Davidson, Toye, Berger, Bailey, Gillespie, Williams and Finn.
Conflict of interest statement
AF is a member of the Joint Committee on Vaccination and Immunisation, the UK National Immunisation Technical Advisory Group and is chair of the WHO European Regional Technical Advisory Group of Experts (ETAGE) on immunization and ex officio a member of the WHO SAGE working group on COVID vaccines. He is investigator COVID-19 vaccine on studies and trials funded by Pfizer, Sanofi, Valneva, the Gates Foundation and the UK government. This manuscript presents independent research funded in part by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
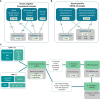
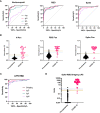

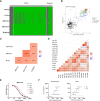

References
-
- COVID-19 weekly epidemiological update. World Health Organization; (2022). Geneva, Switzerland. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
-
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet (2021) 397(10283):1459–69. doi: 10.1016/S0140-6736(21)00675-9 - DOI - PMC - PubMed
-
- Jonczyk R, Stanislawski N, Seiler LK, Blume H, Heiden S, Lucas H, et al. Combined prospective seroconversion and PCR data of selected cohorts indicate a high rate of subclinical SARS-CoV-2 infections-an open observational study in lower Saxony, Germany. Microbiol Spectr. (2022) 10(1):e0151221–e. doi: 10.1128/spectrum.01512-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

