Dietary sugars modulate bacterial-fungal interactions in saliva and inter-kingdom biofilm formation on apatitic surface
- PMID: 36439211
- PMCID: PMC9681999
- DOI: 10.3389/fcimb.2022.993640
Dietary sugars modulate bacterial-fungal interactions in saliva and inter-kingdom biofilm formation on apatitic surface
Abstract
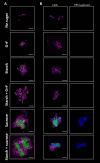
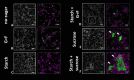
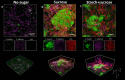
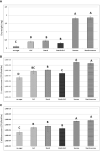
Bacteria and fungi can interact to form inter-kingdom biofilms in the oral cavity. Streptococcus mutans and Candida albicans are frequently detected in saliva and in dental biofilms associated with early childhood caries (tooth-decay), a prevalent oral disease induced by dietary sugars. However, how different sugars influence this bacterial-fungal interaction remains unclear. Here, we investigate whether specific sugars affect the inter-kingdom interaction in saliva and subsequent biofilm formation on tooth-mimetic surfaces. The microbes were incubated in saliva containing common dietary sugars (glucose and fructose, sucrose, starch, and combinations) and analyzed via fluorescence imaging and quantitative computational analyses. The bacterial and fungal cells in saliva were then transferred to hydroxyapatite discs (tooth mimic) to allow microbial binding and biofilm development. We found diverse bacterial-fungal aggregates which varied in size, structure, and spatial organization depending on the type of sugars. Sucrose and starch+sucrose induced the formation of large mixed-species aggregates characterized by bacterial clusters co-bound with fungal cells, whereas mostly single-cells were found in the absence of sugar or in the presence of glucose and fructose. Notably, both colonization and further growth on the apatitic surface were dependent on sugar-mediated aggregation, leading to biofilms with distinctive spatial organizations and 3D architectures. Starch+sucrose and sucrose-mediated aggregates developed into large and highly acidogenic biofilms with complex network of bacterial and fungal cells (yeast and hyphae) surrounded by an intricate matrix of extracellular glucans. In contrast, biofilms originated from glucose and fructose-mediated consortia (or without sugar) were sparsely distributed on the surface without structural integration, growing predominantly as individual species with reduced acidogenicity. These findings reveal the impact of dietary sugars on inter-kingdom interactions in saliva and how they mediate biofilm formation with distinctive structural organization and varying acidogenicity implicated with human tooth-decay.
Keywords: C. albicans; EPS; S. mutans; inter-kingdom aggregate; saliva; sucrose.
Copyright © 2022 Negrini, Ren, Miao, Kim, Simon-Soro, Liu, Koo and Arthur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Influences of starch and sucrose on Streptococcus mutans biofilms.Oral Microbiol Immunol. 2008 Jun;23(3):206-12. doi: 10.1111/j.1399-302X.2007.00412.x. Oral Microbiol Immunol. 2008. PMID: 18402606
-
Addition of cariogenic pathogens to complex oral microflora drives significant changes in biofilm compositions and functionalities.Microbiome. 2023 Jun 1;11(1):123. doi: 10.1186/s40168-023-01561-7. Microbiome. 2023. PMID: 37264481 Free PMC article.
-
Understanding the Basis of METH Mouth Using a Rodent Model of Methamphetamine Injection, Sugar Consumption, and Streptococcus mutans Infection.mBio. 2021 Mar 9;12(2):e03534-20. doi: 10.1128/mBio.03534-20. mBio. 2021. PMID: 33688011 Free PMC article.
-
Oral fungal-bacterial biofilm models in vitro: a review.Med Mycol. 2018 Aug 1;56(6):653-667. doi: 10.1093/mmy/myx111. Med Mycol. 2018. PMID: 29228383 Review.
-
In it together: Candida-bacterial oral biofilms and therapeutic strategies.Environ Microbiol Rep. 2022 Apr;14(2):183-196. doi: 10.1111/1758-2229.13053. Epub 2022 Feb 26. Environ Microbiol Rep. 2022. PMID: 35218311 Free PMC article. Review.
Cited by
-
The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status.Nutrients. 2024 Apr 10;16(8):1113. doi: 10.3390/nu16081113. Nutrients. 2024. PMID: 38674804 Free PMC article.
-
Decoding Early Childhood Caries: A Comprehensive Review Navigating the Impact of Evolving Dietary Trends in Preschoolers.Cureus. 2024 Apr 13;16(4):e58170. doi: 10.7759/cureus.58170. eCollection 2024 Apr. Cureus. 2024. PMID: 38741840 Free PMC article. Review.
-
Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: a literature review.Front Cell Infect Microbiol. 2023 May 16;13:1151532. doi: 10.3389/fcimb.2023.1151532. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37260705 Free PMC article. Review.
-
Measurement and analysis of microbial fluoride resistance in dental biofilm models.Methods Enzymol. 2024;696:155-174. doi: 10.1016/bs.mie.2023.12.018. Epub 2024 Feb 2. Methods Enzymol. 2024. PMID: 38658078 Free PMC article.
-
DMAHDM@MPC nanoparticles in orthodontic adhesive inhibit cariogenic bacteria and sugar metabolism to prevent enamel demineralization.Mater Today Bio. 2025 Jun 9;33:101969. doi: 10.1016/j.mtbio.2025.101969. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40547485 Free PMC article.
References
-
- Botelho J. N., Villegas-Salinas M., Troncoso-Gajardo P., Giacaman R. A., Cury J. A. (2016). Enamel and dentine demineralization by a combination of starch and sucrose in a biofilm - caries model. Braz. Oral. Res. 30 (1), S1806–83242016000100250. doi: 10.1590/1807-3107BOR-2016.vol30.0052 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

