Imbalance hepatic metabolism homeostasis in the F1 generation of endometrial DNMT3B conditional knockout female mice
- PMID: 36439251
- PMCID: PMC9692016
- DOI: 10.3389/fphys.2022.1042449
Imbalance hepatic metabolism homeostasis in the F1 generation of endometrial DNMT3B conditional knockout female mice
Abstract
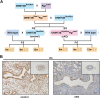
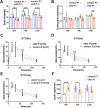
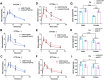
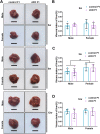
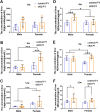
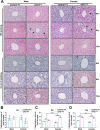
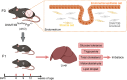
Numerous studies have suggested the possibility of explaining the etiology of metabolic syndrome through DNA methylation. DNA methyltransferase 3B (DNMT3B) plays an important role in de novo DNA methylation. There was an alteration in maternal (F0) endometrial function, which might lead to growth and developmental disorder in offspring (F1). In this study, we investigated the effect of maternal endometrial DNMT3B deficiency on the metabolism in offspring. We constructed endometrial DNMT3B conditional knockout female mice (cKO) which were mated with normal C57BL/6 male mice to obtain the F1 generation. Further, to study the development of these offspring, we observed them at three different life stages which included the 6-week-old juvenile, 9-week-old sub-adult and 12-week-old adult. Follow the detection of a range of metabolism-related indicators, we found that in the cKO F1 generation, liver triglyceride level was significantly elevated in 9-week-old female mice, lipid droplet deposition was significantly increased in 9-week-old and 12-week-old mice, and the expression of lipid metabolism key factors in the liver was markedly decreased except of 6-week-old male mice. These results indicate that maternal endometrial DNMT3B conditional knockout leads to imbalance in hepatic metabolism in F1 generation, the mechanism of which requires further discussion.
Keywords: DNMT3B; F1 generation; glucose; lipid metabolism; liver.
Copyright © 2022 Li, Gao, Ding, Chen, Liu, He, Li, Long, Lu, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Anagnostis P., Christou K., Artzouchaltzi A. M., Gkekas N. K., Kosmidou N., Siolos P., et al. (2019). Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Endocrinol. 180, 41–50. 10.1530/EJE-18-0602 - DOI - PubMed
-
- Castellano-Castillo D., Moreno-Indias I., Sanchez-Alcoholado L., Ramos-Molina B., Alcaide-Torres J., Morcillo S., et al. (2019). Altered adipose tissue DNA methylation status in metabolic syndrome: Relationships between global DNA methylation and specific methylation at adipogenic, lipid metabolism and inflammatory candidate genes and metabolic variables. J. Clin. Med. 8, 87. 10.3390/jcm8010087 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

