Targeting the MDM2-p53 pathway in dedifferentiated liposarcoma
- PMID: 36439412
- PMCID: PMC9684653
- DOI: 10.3389/fonc.2022.1006959
Targeting the MDM2-p53 pathway in dedifferentiated liposarcoma
Abstract
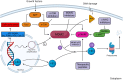
Dedifferentiated liposarcoma (DDLPS) is an aggressive adipogenic cancer with poor prognosis. DDLPS tumors are only modestly sensitive to chemotherapy and radiation, and there is a need for more effective therapies. Genetically, DDLPS is characterized by a low tumor mutational burden and frequent chromosomal structural abnormalities including amplification of the 12q13-15 chromosomal region and the MDM2 gene, which are defining features of DDLPS. The MDM2 protein is an E3 ubiquitin ligase that targets the tumor suppressor, p53, for proteasomal degradation. MDM2 amplification or overexpression in human malignancies is associated with cell-cycle progression and worse prognosis. The MDM2-p53 interaction has thus garnered interest as a therapeutic target for DDLPS and other malignancies. MDM2 binds p53 via a hydrophobic protein interaction that is easily accessible with synthetic analogues. Multiple agents have been developed, including Nutlins such as RG7112 and small molecular inhibitors including SAR405838 and HDM201. Preclinical in vitro and animal models have shown promising results with MDM2 inhibition, resulting in robust p53 reactivation and cancer cell death. However, multiple early-phase clinical trials have failed to show a benefit with MDM2 pathway inhibition for DDLPS. Mechanisms of resistance are being elucidated, and novel inhibitors and combination therapies are currently under investigation. This review provides an overview of these strategies for targeting MDM2 in DDLPS.
Keywords: MDM2; clinical trial; dedifferentiated liposarcoma; p53; small molecular inhibitor; targeted therapy.
Copyright © 2022 Traweek, Cope, Roland, Keung, Nassif and Erstad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

