Salinity and hydraulic retention time induce membrane phospholipid acyl chain remodeling in Halanaerobium congolense WG10 and mixed cultures from hydraulically fractured shale wells
- PMID: 36439785
- PMCID: PMC9687094
- DOI: 10.3389/fmicb.2022.1023575
Salinity and hydraulic retention time induce membrane phospholipid acyl chain remodeling in Halanaerobium congolense WG10 and mixed cultures from hydraulically fractured shale wells
Abstract
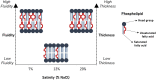
Bacteria remodel their plasma membrane lipidome to maintain key biophysical attributes in response to ecological disturbances. For Halanaerobium and other anaerobic halotolerant taxa that persist in hydraulically fractured deep subsurface shale reservoirs, salinity, and hydraulic retention time (HRT) are important perturbants of cell membrane structure, yet their effects remain poorly understood. Membrane-linked activities underlie in situ microbial growth kinetics and physiologies which drive biogeochemical reactions in engineered subsurface systems. Hence, we used gas chromatography-mass spectrometry (GC-MS) to investigate the effects of salinity and HRT on the phospholipid fatty acid composition of H. congolense WG10 and mixed enrichment cultures from hydraulically fractured shale wells. We also coupled acyl chain remodeling to membrane mechanics by measuring bilayer elasticity using atomic force microscopy (AFM). For these experiments, cultures were grown in a chemostat vessel operated in continuous flow mode under strict anoxia and constant stirring. Our findings show that salinity and HRT induce significant changes in membrane fatty acid chemistry of H. congolense WG10 in distinct and complementary ways. Notably, under nonoptimal salt concentrations (7% and 20% NaCl), H. congolense WG10 elevates the portion of polyunsaturated fatty acids (PUFAs) in its membrane, and this results in an apparent increase in fluidity (homeoviscous adaptation principle) and thickness. Double bond index (DBI) and mean chain length (MCL) were used as proxies for membrane fluidity and thickness, respectively. These results provide new insight into our understanding of how environmental and engineered factors might disrupt the physical and biogeochemical equilibria of fractured shale by inducing physiologically relevant changes in the membrane fatty acid chemistry of persistent microbial taxa. GRAPHICAL ABSTRACTSalinity significantly alters membrane bilayer fluidity and thickness in Halanaerobium congolense WG10.
Keywords: Halanaerobium; fatty acid methyl ester; fractured shale; hydraulic retention time; lipids; membrane adaptation; salinity.
Copyright © 2022 Ugwuodo, Colosimo, Adhikari, Shen, Badireddy and Mouser.
Conflict of interest statement
FC is currently employed by New England Biolabs, Ipswich, MA, United States. JA is employed by Sanborn, Head and Associates Inc., Concord, NH, United States. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Changes in environmental and engineered conditions alter the plasma membrane lipidome of fractured shale bacteria.Microbiol Spectr. 2024 Jan 11;12(1):e0233423. doi: 10.1128/spectrum.02334-23. Epub 2023 Dec 7. Microbiol Spectr. 2024. PMID: 38059585 Free PMC article.
-
Aromatic amino acid metabolism and active transport regulation are implicated in microbial persistence in fractured shale reservoirs.ISME Commun. 2024 Nov 26;4(1):ycae149. doi: 10.1093/ismeco/ycae149. eCollection 2024 Jan. ISME Commun. 2024. PMID: 39670059 Free PMC article.
-
Deep-Subsurface Pressure Stimulates Metabolic Plasticity in Shale-Colonizing Halanaerobium spp.Appl Environ Microbiol. 2019 May 30;85(12):e00018-19. doi: 10.1128/AEM.00018-19. Print 2019 Jun 15. Appl Environ Microbiol. 2019. PMID: 30979840 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Understanding hydraulic fracturing: a multi-scale problem.Philos Trans A Math Phys Eng Sci. 2016 Oct 13;374(2078):20150426. doi: 10.1098/rsta.2015.0426. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27597789 Free PMC article. Review.
Cited by
-
The response of soil microbial community to application of organic amendment to saline land.Front Microbiol. 2025 Jan 6;15:1481156. doi: 10.3389/fmicb.2024.1481156. eCollection 2024. Front Microbiol. 2025. PMID: 39834373 Free PMC article.
-
Changes in environmental and engineered conditions alter the plasma membrane lipidome of fractured shale bacteria.Microbiol Spectr. 2024 Jan 11;12(1):e0233423. doi: 10.1128/spectrum.02334-23. Epub 2023 Dec 7. Microbiol Spectr. 2024. PMID: 38059585 Free PMC article.
References
-
- Abbas G., Saqib M., Akhtar J., Haq M. A. (2015). Interactive effects of salinity and iron deficiency on different rice genotypes. J. Plant Nutr. Soil Sci. 178, 306–311. doi: 10.1002/jpln.201400358 - DOI
-
- Baker L. Y., Hobby C. R., Siv A. W., Bible W. C., Glennon M. S., Anderson D. M., et al. . (2018). Pseudomonas aeruginosa responds to exogenous polyunsaturated fatty acids (PUFAs) by modifying phospholipid composition, membrane permeability, and phenotypes associated with virulence. BMC Microbiol. 18:117. doi: 10.1186/s12866-018-1259-8, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

