Vancomycin-induced gut microbiota dysbiosis aggravates allergic rhinitis in mice by altered short-chain fatty acids
- PMID: 36439824
- PMCID: PMC9687373
- DOI: 10.3389/fmicb.2022.1002084
Vancomycin-induced gut microbiota dysbiosis aggravates allergic rhinitis in mice by altered short-chain fatty acids
Abstract
Objective: This study aims to explore how gut microbiota dysbiosis affects allergic rhinitis (AR) and whether short-chain fatty acids (SCFAs) play a role in this process.
Methods: A mouse gut microbiota dysbiosis model was established by adding vancomycin to drinking water for 2 weeks before ovalbumin (OVA) sensitization. Then an OVA-alum AR mouse model was established by intraperitoneal OVA injection followed by nasal excitation. Hematoxylin and eosin (H&E) staining was performed to observe pathological changes in nasal and colon tissues of AR mice. Serum levels of total-IgE, OVA-sIgE, IL-4, IL-5, IL-10, and TGF-β1 were measured. The composition and diversity of the mouse gut microbiota were observed by 16S rDNA sequencing. Levels of SCFAs in feces were determined using SCFA-targeted metabolomics. Sodium butyrate (NaB) was added daily to mice on a low-fiber basal diet 2 weeks before the first sensitization, until the end of the study.
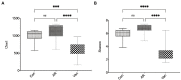
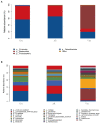
Results: After gut microbiota dysbiosis, serum levels of the total IgE, OVA-sIgE, IL-4, and IL-5 in AR mice were significantly increased, compared with the control group. The composition and diversity of gut microbiota were significantly altered after gut microbiota dysbiosis, with the fecal SCFAs significantly reduced as well. The reduced bacterial genera after gut microbiota dysbiosis, such as Ruminococcus and Lactobacillus, were significantly and positively correlated with SCFAs. In contrast, the increased genera in the Van group, such as Escherichia-Shigella and Klebsiella, were significantly negatively correlated with SCFAs in feces. NaB treatment significantly reduced total-IgE, OVA-sIgE, IL-4, and IL-5 levels in serum, and inflammatory infiltration of the nasal and colon mucosa. In addition, serum levels of IL-10 and TGF-β1 increased significantly after NaB treatment. Foxp3 protein in the colon was upregulated considerably after NaB intervention.
Conclusion: Vancomycin-induced gut microbiota dysbiosis increased susceptibility and severity of AR, which is significantly related to reduced SCFA-producing bacteria, fecal SCFAs, and specific bacterial taxa. In addition, it was found that NaB alleviated low dietary fiber base-fed symptoms and immune status in AR mice.
Keywords: allergic rhinitis; butyrate; gut microbiota dysbiosis; short-chain fatty acids; tregs.
Copyright © 2022 Chen, Xu, Liu, Wei, He, Lin, Wang, Li and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures












References
-
- Agus A., Denizot J., Thévenot J., Martinez-Medina M., Massier S., Sauvanet P., et al. (2016). Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 6:19032. doi: 10.1038/srep19032, PMID: - DOI - PMC - PubMed
-
- Andreassen M., Rudi K., Angell I. L., Dirven H., Nygaard U. C. (2018). Allergen immunization induces major changes in microbiota composition and short-chain fatty acid production in different gut segments in a mouse model of lupine food allergy. Int. Arch. Allergy Immunol. 177, 311–323. doi: 10.1159/000492006, PMID: - DOI - PubMed
-
- Bianchi F., Dall Asta M., Del Rio D., Mangia A., Musci M., Scazzina F. (2011). Development of a headspace solid-phase microextraction gas chromatography–mass spectrometric method for the determination of short-chain fatty acids from intestinal fermentation. Food Chem. 129, 200–205. doi: 10.1016/j.foodchem.2011.04.022 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

