Catalytic asymmetric synthesis of carbocyclic C-nucleosides
- PMID: 36439888
- PMCID: PMC9676730
- DOI: 10.1038/s42004-022-00773-6
Catalytic asymmetric synthesis of carbocyclic C-nucleosides
Abstract
Access to carbocyclic C-nucleosides (CC-Ns) is currently restricted. The few methods available to make CC-Ns suffer from long syntheses and poor modularity, hindering the examination of potentially important chemical space. Here we report an approach to CC-Ns which uses an asymmetric Suzuki-Miyaura type reaction as the key C-C bond forming step. After coupling the densely functionalized racemic bicyclic allyl chloride and heterocyclic boronic acids, the trisubstituted cyclopentenyl core is elaborated to RNA analogues via a hydroborylation-homologation-oxidation sequence. We demonstrate that the approach can be used to produce a variety of enantiomerically enriched CC-Ns, including a carbocyclic derivative of Showdomycin.
Keywords: Asymmetric catalysis; Asymmetric synthesis; Synthetic chemistry methodology.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



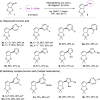

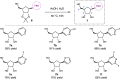



References
-
- Sofia, M. J. Nucleosides and Nucleotides for the Treatment of Viral Diseases. In Annual Reports in Medicinal Chemistry. Vol. 49 Ch. 221, 221–247 (Elsevier, 2014).
-
- Singh S, Bhattarai D, Veeraswamy G, Choi Y, Lee K. Nucleosides with modified sugar ring: synthesis and biological activities. Curr. Org. Chem. 2016;20:856–897. doi: 10.2174/1385272819666150803235458. - DOI
-
- Agrofoglio, L. A. & Challand, S. R. Biological activity of carbocyclic nucleosides. In Acyclic, Carbocyclic L-Nucleosides. Ch. 5, 256–284 (Springer, 1998).
-
- Chu, D. & Rao, J. Carbocyclic compounds and methods for treating emerging diseases, including Influenza and Venezuela Equine Encephalitis virus. WO2008124157A1 (2008).
LinkOut - more resources
Full Text Sources
Miscellaneous

