CD44 Receptor-Targeted and Reactive Oxygen Species-Responsive H2S Donor Micelles Based on Hyaluronic Acid for the Therapy of Renal Ischemia/Reperfusion Injury
- PMID: 36440107
- PMCID: PMC9686187
- DOI: 10.1021/acsomega.2c05407
CD44 Receptor-Targeted and Reactive Oxygen Species-Responsive H2S Donor Micelles Based on Hyaluronic Acid for the Therapy of Renal Ischemia/Reperfusion Injury
Abstract
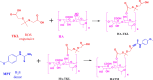
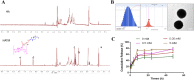
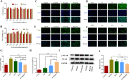
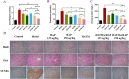
For the therapy attenuating renal ischemia-reperfusion (IR) injury, a novel drug delivery system was urgently needed, which could precisely deliver drugs to the pathological renal tissue. Here, we have prepared new nanomaterials with a reactive oxygen species (ROS)-responsive hydrogen sulfide (H2S) donor and hyaluronic acid that targets CD44 receptor. The novel material was synthesized and characterized via related experiments. Then, rapamycin was loaded, which inhibited kidney damage. In the in vitro study, we found that the micelles had ROS-responsiveness, biocompatibility, and cell penetration. In addition, the experimental results showed that the intracellular H2S concentration after administration was threefold higher than that of the control group. The western blot assay revealed that they have anti-inflammatory effects via H2S donor blocking the NF-κB signaling pathway. Consequently, the rising CD44 receptor-targeting and ROS-sensitive H2S donor micelles would provide a promising way for renal IR injury. This work provides a strategy for improving ischemia/reperfusion injury for pharmaceuticals.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Novel dual ROS-sensitive and CD44 receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis.Carbohydr Polym. 2020 Mar 15;232:115787. doi: 10.1016/j.carbpol.2019.115787. Epub 2019 Dec 26. Carbohydr Polym. 2020. PMID: 31952595
-
Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway.Eur J Pharmacol. 2014 Feb 15;725:70-8. doi: 10.1016/j.ejphar.2014.01.009. Epub 2014 Jan 18. Eur J Pharmacol. 2014. PMID: 24444438
-
Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats.Ren Fail. 2015 May;37(4):727-33. doi: 10.3109/0886022X.2015.1012983. Epub 2015 Feb 20. Ren Fail. 2015. PMID: 25697231
-
A CRITICAL REVIEW ON PHARMACOLOGICAL SIGNIFICANCE OF HYDROGEN SULFIDE (H₂S) ON NF-κB CONCENTRATION AND ICAM-1 EXPRESSION IN RENAL ISCHEMIA REPERFUSION INJURY.Acta Pol Pharm. 2017 May;74(3):747-752. Acta Pol Pharm. 2017. PMID: 29513943 Review.
-
H2S donor molecules against cold ischemia-reperfusion injury in preclinical models of solid organ transplantation.Pharmacol Res. 2021 Oct;172:105842. doi: 10.1016/j.phrs.2021.105842. Epub 2021 Aug 24. Pharmacol Res. 2021. PMID: 34450311 Review.
Cited by
-
Hyaluronidase inhibitor sHA2.75 alleviates ischemia-reperfusion-induced acute kidney injury.Cell Cycle. 2024 Feb;23(3):248-261. doi: 10.1080/15384101.2024.2309019. Epub 2024 Mar 25. Cell Cycle. 2024. PMID: 38526145 Free PMC article.
References
-
- Cao H.; Cheng Y.; Gao H.; Zhuang J.; Zhang W.; Bian Q.; Wang F.; Du Y.; Li Z.; Kong D.; et al. In Vivo Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondrial Function in Renal Ischemia-Reperfusion Injury. ACS Nano 2020, 14, 4014–4026. 10.1021/acsnano.9b08207. - DOI - PubMed
- Dwyer K. M.; Kishore B. K.; Robson S. C. Conversion of extracellular ATP into adenosine: a master switch in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 509–524. 10.1038/s41581-020-0304-7. - DOI - PubMed
- Huang C. L.; Zeng T.; Li J. W.; Tan L. S.; Deng X. L.; Pan Y. C.; Chen Q.; Li A. Q.; Hu J. Q. Folate Receptor-Mediated Renal-Targeting Nanoplatform for the Specific Delivery of Triptolide to Treat Renal Ischemia/Reperfusion Injury. ACS Biomater. Sci. Eng. 2019, 5, 2877–2886. 10.1021/acsbiomaterials.9b00119. - DOI - PubMed
- Wang S.; Liu A.; Wu G.; Ding H. F.; Huang S.; Nahman S.; Dong Z. The CPLANE protein Intu protects kidneys from ischemia-reperfusion injury by targeting STAT1 for degradation. Nat. Commun. 2018, 9, 1234.10.1038/s41467-018-03628-8. - DOI - PMC - PubMed
-
- Carcy R.; Cougnon M.; Poet M.; Durandy M.; Sicard A.; Counillon L.; Blondeau N.; Hauet T.; Tauc M. Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radical Biol. Med. 2021, 169, 258–270. 10.1016/j.freeradbiomed.2021.04.023. - DOI - PubMed
- Roorda M.; Miljkovic J. L.; van Goor H.; Henning R. H.; Bouma H. R. Spatiotemporal regulation of hydrogen sulfide signaling in the kidney. Redox Biol. 2021, 43, 10196110.1016/j.redox.2021.101961. - DOI - PMC - PubMed
-
- T P. M. K.; Nikolic-Paterson D. J.; Lan H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. 10.1038/s41581-019-0110-2. - DOI - PubMed
- Vegting Y.; Vogt L.; Anders H. J.; De Winther M. P. J.; Bemelman F. J.; Hilhorst M. L. Monocytes and macrophages in ANCA-associated vasculitis. Autoimmun. Rev. 2021, 20, 10291110.1016/j.autrev.2021.102911. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

