Tetrathiafulvalene-Benzothiadiazole: A Metal-Free Photocatalyst for Hydrogen Production
- PMID: 36440178
- PMCID: PMC9685743
- DOI: 10.1021/acsomega.2c05185
Tetrathiafulvalene-Benzothiadiazole: A Metal-Free Photocatalyst for Hydrogen Production
Abstract
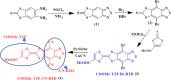
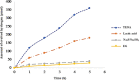
In this work, a series of hybrid tetrathiafulvalene-benzothiadiazole (TTF-BTD) are designed and applied as a metal-free photocatalyst for hydrogen production, particularly under visible light irradiation. Density functional theory calculations are used to shed light on the photophysical properties observed in the various TTF-BTD derivatives and investigated by the obtained data. Because band gap engineering has normally been used as an effective approach, we studied the effect of the various functional groups on the band gap to set a favorable band alignment with photocatalysts. An increase in highest occupied molecular orbital and lowest unoccupied molecular orbital energy levels is observed in the order CH3 < Br < CF3 < COOMe < CN. The results discover that COOMe-TTF-CN-BTD can have a clear photocatalytic potential in the hydrogen production for specific applications. Our experimental and theoretical studies reveal that the CN-withdrawing group increases the reduction potential of the conduction band; meanwhile, COOMe decreases the reduction potential of the valance band. Moreover, we demonstrate that H2O reduction and oxidation reaction energies are both located inside the COOMe-TTF-CN-BTD band gap that enables an enhanced photocatalytic hydrogen evolution rate of 122 μmol h-1 g-1 under visible light. The efficiency of the COOMe-TTF-CN-BTD photocatalyst is also described in terms of medium pH and the nature of the sacrificial agent, where the maximum hydrogen production efficiency is observed at high pH. The findings point to a means of efficient production of hydrogen that can be directly achieved under visible light irradiation without any modifications.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Cao H.; Zhang Z.; Zhang M.; Gu A.; Yu H.; Ban H.; Sun Q.; Shen Y.; Zhang X.-L.; Zhu J.; Wang M. The effect of defects in tin-based perovskites and their photovoltaic devices. Mater. Today Phys. 2021, 21, 100513.10.1016/j.mtphys.2021.100513. - DOI
-
- Luo D.; Ren Z. Synthesis of sodium nanoparticles for promising extraction of heavy oil. Mater. Today Phys. 2021, 16, 100276.10.1016/j.mtphys.2020.100276. - DOI
-
- Bartela Ł. A hybrid energy storage system using compressed air and hydrogen as the energy carrier. Energy 2020, 196, 117088.10.1016/j.energy.2020.117088. - DOI
-
- Sazali N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. 10.1016/j.ijhydene.2020.05.021. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

