Histological and serological features of acute liver injury after SARS-CoV-2 vaccination
- PMID: 36440259
- PMCID: PMC9691430
- DOI: 10.1016/j.jhepr.2022.100605
Histological and serological features of acute liver injury after SARS-CoV-2 vaccination
Abstract
Background & aims: Liver injury with autoimmune features after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is increasingly reported. We investigated a large international cohort of individuals with acute hepatitis arising after SARS-CoV-2 vaccination, focusing on histological and serological features.
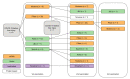
Methods: Individuals without known pre-existing liver diseases and transaminase levels ≥5x the upper limit of normal within 3 months after any anti-SARS-CoV-2 vaccine, and available liver biopsy were included. Fifty-nine patients were recruited; 35 females; median age 54 years. They were exposed to various combinations of mRNA, vectorial, inactivated and protein-based vaccines.
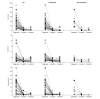
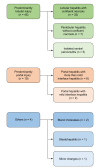
Results: Liver histology showed predominantly lobular hepatitis in 45 (76%), predominantly portal hepatitis in 10 (17%), and other patterns in four (7%) cases; seven had fibrosis Ishak stage ≥3, associated with more severe interface hepatitis. Autoimmune serology, centrally tested in 31 cases, showed anti-antinuclear antibody in 23 (74%), anti-smooth muscle antibody in 19 (61%), anti-gastric parietal cells in eight (26%), anti-liver kidney microsomal antibody in four (13%), and anti-mitochondrial antibody in four (13%) cases. Ninety-one percent were treated with steroids ± azathioprine. Serum transaminase levels improved in all cases and were normal in 24/58 (41%) after 3 months, and in 30/46 (65%) after 6 months. One patient required liver transplantation. Of 15 patients re-exposed to SARS-CoV-2 vaccines, three relapsed.
Conclusion: Acute liver injury arising after SARS-CoV-2 vaccination is frequently associated with lobular hepatitis and positive autoantibodies. Whether there is a causal relationship between liver damage and SARS-CoV-2 vaccines remains to be established. A close follow-up is warranted to assess the long-term outcomes of this condition.
Impact and implications: Cases of liver injury after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) have been published. We investigated a large international cohort of individuals with acute hepatitis after SARS-CoV-2 vaccination, focusing on liver biopsy findings and autoantibodies: liver biopsy frequently shows inflammation of the lobule, which is typical of recent injury, and autoantibodies are frequently positive. Whether there is a causal relationship between liver damage and SARS-CoV-2 vaccines remains to be established. Close follow-up is warranted to assess the long-term outcome of this condition.
Keywords: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibody; ANA, anti-nuclear antibody; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; DILI, drug-induced liver injury; IAIHG, International Autoimmune Hepatitis Group; IFT, indirect immunofluorescence; LKM, liver kidney microsomal; LT, liver transplantation; PCA, parietal cell antigen; SARS-CoV-2 vaccines; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SLA, soluble liver antigen; SMA, anti-smooth muscle antibody; ULN, upper limit of normal; acute liver injury; autoimmune liver serology; liver histology; pIgG, polyreactive IgG.
© 2022 The Author(s).
Conflict of interest statement
The authors do not have any conflict of interest pertaining to this study. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

