Changes in prenatal testing during the COVID-19 pandemic
- PMID: 36440341
- PMCID: PMC9682111
- DOI: 10.3389/fped.2022.1064039
Changes in prenatal testing during the COVID-19 pandemic
Abstract
Objective: The coronavirus disease 2019 (COVID-19) pandemic disrupted healthcare delivery, including prenatal care. The study objective was to assess if timing of routine prenatal testing changed during the COVID-19 pandemic.
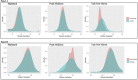
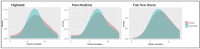
Methods: Retrospective observational cohort study using claims data from a regional insurer (Highmark) and electronic health record data from two academic health systems (Penn Medicine and Yale New Haven) to compare prenatal testing timing in the pre-pandemic (03/10/2018-12/31/2018 and 03/10/2019-12/31/2019) and early COVID-19 pandemic (03/10/2020-12/31/2020) periods. Primary outcomes were second trimester fetal anatomy ultrasounds and gestational diabetes (GDM) testing. A secondary analysis examined first trimester ultrasounds.
Results: The three datasets included 31,474 pregnant patients. Mean gestational age for second trimester anatomy ultrasounds increased from the pre-pandemic to COVID-19 period (Highmark 19.4 vs. 19.6 weeks; Penn: 20.1 vs. 20.4 weeks; Yale: 18.8 vs. 19.2 weeks, all p < 0.001). There was a detectable decrease in the proportion of patients who completed the anatomy survey <20 weeks' gestation across datasets, which did not persist at <23 weeks' gestation. There were no consistent changes in timing of GDM screening. There were significant reductions in the proportion of patients with first trimester ultrasounds in the academic institutions (Penn: 57.7% vs. 40.6% and Yale: 78.7% vs. 65.5%, both p < 0.001) but not Highmark. Findings were similar with multivariable adjustment.
Conclusion: While some prenatal testing happened later in pregnancy during the pandemic, pregnant patients continued to receive appropriately timed testing. Despite disruptions in care delivery, prenatal screening remained a priority for patients and providers during the COVID-19 pandemic.
Keywords: COVID-19; access to care; gestational diabetes; glucose tolerance test; pregnancy; prenatal care; ultrasound.
© 2022 Handley, Ledyard, Lundsberg, Passarella, Yang, Son, Mckenney, Greenspan, Dysart, Culhane and Burris.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Reddy UM, Abuhamad AZ, Levine D, Saade GR. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver national institute of child health and human development, society for maternal-fetal medicine, American institute of ultrasound in medicine, American college of obstetricians and gynecologists, American college of radiology, society for pediatric radiology, and society of radiologists in ultrasound fetal imaging workshop. Obstet Gynecol. (2014) 123:1070–82. 10.1097/AOG.0000000000000245 - DOI - PubMed
LinkOut - more resources
Full Text Sources