Stability and conformational memory of electrosprayed and rehydrated bacteriophage MS2 virus coat proteins
- PMID: 36440379
- PMCID: PMC9685359
- DOI: 10.1016/j.crstbi.2022.10.001
Stability and conformational memory of electrosprayed and rehydrated bacteriophage MS2 virus coat proteins
Abstract
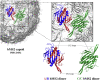
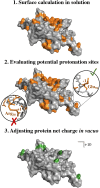
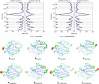
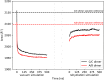
Proteins are innately dynamic, which is important for their functions, but which also poses significant challenges when studying their structures. Gas-phase techniques can utilise separation and a range of sample manipulations to transcend some of the limitations of conventional techniques for structural biology in crystalline or solution phase, and isolate different states for separate interrogation. However, the transfer from solution to the gas phase risks affecting the structures, and it is unclear to what extent different conformations remain distinct in the gas phase, and if resolution in silico can recover the native conformations and their differences. Here, we use extensive molecular dynamics simulations to study the two distinct conformations of dimeric capsid protein of the MS2 bacteriophage. The protein undergoes notable restructuring of its peripheral parts in the gas phase, but subsequent simulation in solvent largely recovers the native structure. Our results suggest that despite some structural loss due to the experimental conditions, gas-phase structural biology techniques provide meaningful data that inform not only about the structures but also conformational dynamics of proteins.
Keywords: Bacteriophage; Electrospray ionization; Gas-phase structure; Molecular dynamics simulations; Protein structure; Solvation.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Abramsson M.L., Sahin C., Hopper J.T.S., Branca R.M.M., Danielsson J., Xu M., Chandler S.A., Österlund N., Ilag L.L., Leppert A., Costeira-Paulo J., Lang L., Teilum K., Laganowsky A., Benesch J.L.P., Oliveberg M., Robinson C.V., Marklund E.G., Allison T.M., Winther J.R., Landreh M. Charge engineering reveals the roles of ionizable side chains in electrospray ionization mass spectrometry. JACS Au. 2021;1:2385–2393. doi: 10.1021/jacsau.1c00458. - DOI - PMC - PubMed
-
- Abramsson M.L., Sahin C., Hopper J.T.S., Branca R.M.M., Danielsson J., Xu M., Chandler S.A., Österlund N., Ilag L.L., Leppert A., Costeira-Paulo J., Lang L., Teilum K., Laganowsky A., Benesch J.L.P., Oliveberg M., Robinson C.V., Marklund E.G., Allison T.M., Winther J.R., Landreh M. Charge engineering reveals the roles of ionizable side chains in elec-trospray ionization mass spectrometry. JACS Au. 2021;1(12):2385–2393. doi: 10.1021/jacsau.1c00458. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

