Transposon control as a checkpoint for tissue regeneration
- PMID: 36440631
- PMCID: PMC10655923
- DOI: 10.1242/dev.191957
Transposon control as a checkpoint for tissue regeneration
Abstract
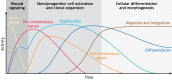
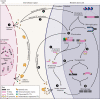
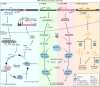
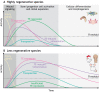
Tissue regeneration requires precise temporal control of cellular processes such as inflammatory signaling, chromatin remodeling and proliferation. The combination of these processes forms a unique microenvironment permissive to the expression, and potential mobilization of, transposable elements (TEs). Here, we develop the hypothesis that TE activation creates a barrier to tissue repair that must be overcome to achieve successful regeneration. We discuss how uncontrolled TE activity may impede tissue restoration and review mechanisms by which TE activity may be controlled during regeneration. We posit that the diversification and co-evolution of TEs and host control mechanisms may contribute to the wide variation in regenerative competency across tissues and species.
Keywords: Inflammation; Proliferation; Regenerative biology; Stem cells; Transposable elements.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
References
-
- Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., Athineos, D., Kang, T.-W., Lasitschka, F., Andrulis, M.et al. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978-990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Alié, A., Leclère, L., Jager, M., Dayraud, C., Chang, P., Guyader, H. L., Quéinnec, E. and Manuel, M. (2011). Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: ancient association of “germline genes” with stemness. Dev. Biol. 350, 183-197. 10.1016/j.ydbio.2010.10.019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

