The formation and function of the neutrophil phagosome
- PMID: 36440666
- PMCID: PMC10952784
- DOI: 10.1111/imr.13173
The formation and function of the neutrophil phagosome
Abstract
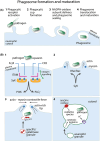
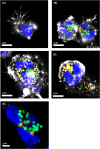
Neutrophils are the most abundant circulating leukocyte and are crucial to the initial innate immune response to infection. One of their key pathogen-eliminating mechanisms is phagocytosis, the process of particle engulfment into a vacuole-like structure called the phagosome. The antimicrobial activity of the phagocytic process results from a collaboration of multiple systems and mechanisms within this organelle, where a complex interplay of ion fluxes, pH, reactive oxygen species, and antimicrobial proteins creates a dynamic antimicrobial environment. This complexity, combined with the difficulties of studying neutrophils ex vivo, has led to gaps in our knowledge of how the neutrophil phagosome optimizes pathogen killing. In particular, controversy has arisen regarding the relative contribution and integration of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-derived antimicrobial agents and granule-delivered antimicrobial proteins. Clinical syndromes arising from dysfunction in these systems in humans allow useful insight into these mechanisms, but their redundancy and synergy add to the complexity. In this article, we review the current knowledge regarding the formation and function of the neutrophil phagosome, examine new insights into the phagosomal environment that have been permitted by technological advances in recent years, and discuss aspects of the phagocytic process that are still under debate.
Keywords: neutrophil; phagocytosis; phagosome.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
Andrew Conway Morris is a member of the scientific advisory board of Cambridge Infection Diagnostics Ltd and reports speaking fees from Boston Scientific.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

