The regulatory role of BMP4 in testicular Sertoli cells of Tibetan sheep
- PMID: 36440761
- PMCID: PMC9838805
- DOI: 10.1093/jas/skac393
The regulatory role of BMP4 in testicular Sertoli cells of Tibetan sheep
Abstract
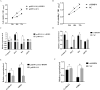
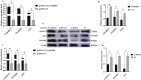
This study aimed to determine the regulatory mechanism of bone morphogenetic protein 4 (BMP4) gene in the testes of Tibetan sheep and its role in the blood-testis barrier (BTB). First, we cloned BMP4 gene for bioinformatics analysis, and detected the mRNA and protein expression levels of BMP4 in the testes of Tibetan sheep pre-puberty (3-mo-old), during sexual maturity (1-yr-old), and in adulthood (3-yr-old) by qRT-PCR and Western blot. In addition, the subcellular localization of BMP4 was analyzed by immunohistochemical staining. Next, BMP4 overexpression and silencing vectors were constructed and transfected into primary Sertoli cells (SCs) to promote and inhibit the proliferation of BMP4, respectively. Then, CCK-8 was used to detect the proliferation effect of SCs. The expression of BMP4 and downstream genes, pathway receptors, tight junction-related proteins, and cell proliferation and apoptosis-related genes in SCs were studied using qRT-PCR and Western blot. The results revealed that the relative expression of BMP4 mRNA and protein in testicular tissues of 1Y group and 3Y group was dramatically higher than that of 3M group (P < 0.01), and BMP4 protein is mainly located in SCs and Leydig cells at different development stages. The CDS region of the Tibetan sheep BMP4 gene was 1,229 bp. CCK-8 results demonstrated that the proliferation rate of BMP4 was significantly increased in the overexpression group (pc-DNA-3.1(+)-BMP4; P < 0.05). In addition, the mRNA and protein expressions of SMAD5, BMPR1A, and BMPR1B and tight junction-related proteins Claudin11, Occludin, and ZO1 were significantly increased (P < 0.05). The mRNA expression of cell proliferation-related gene Bcl2 was significantly enhanced (P < 0.05), and the expression of GDNF was enhanced (P > 0.05). The mRNA expression of apoptosis-related genes Caspase3 and Bax decreased significantly (P < 0.05), while the mRNA expression of cell cycle-related genes CyclinA2 and CDK2 increased significantly (P < 0.05). It is worth noting that the opposite results were observed after transfection with si-BMP4. In summary, what should be clear from the results reported here is that BMP4 affects testicular development by regulating the Sertoli cells and BTB, thereby modulating the spermatogenesis of Tibetan sheep.
Keywords: Sertoli cells; Tibetan sheep; bone morphogenetic protein 4; testis.
Plain language summary
The fertility of male Tibetan sheep is mainly affected by testicular development and spermatogenesis. In these processes, Sertoli cells (SCs) play a central role and are regulated by a variety of genes and factors. BMP4 is mainly distributed in Sertoli cells, and its expression level increases with age. Overexpression of the BMP4 gene in Tibetan sheep testis SCs revealed elevated expression of BMP4 protein and its downstream genes SMAD5, pathway receptor proteins BMPR1A and BMPR1B; followed by elevated expression levels of cell proliferation-related genes and decreased expression levels of apoptosis-related genes. Meanwhile, the expression of tight junction proteins was also elevated. These results indicate that BMP4 affects testicular development by regulating the Sertoli cells and blood–testis barrier, thereby affecting the spermatogenesis of Tibetan sheep.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Figures







References
-
- Andl, T., Ahn K., Kairo A., Chu E. Y., Wine-Lee L., Reddy S. T., Croft N. J., Cebra-Thomas J. A., Metzger D., Chambon P., . et al.. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131:2257–2268. doi:10.1242/dev.01125 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

