Cost evaluation of the Merlin assay for predicting melanoma sentinel lymph node biopsy metastasis
- PMID: 36440797
- PMCID: PMC10098626
- DOI: 10.1111/ijd.16515
Cost evaluation of the Merlin assay for predicting melanoma sentinel lymph node biopsy metastasis
Abstract
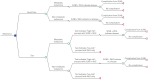
Background: The Merlin assay for melanoma-risk assessment has become commercially available to reduce the rate of unnecessary sentinel lymph node biopsies (SLNB) in SLNB-eligible patients with cutaneous melanoma. Merlin low-risk patients are recommended to undergo wide local excision (WLE) of the primary tumor, whereas Merlin high-risk patients are recommended to undergo both SLNB and WLE. Here, we compared the cost of a Merlin testing strategy to that of a no-testing strategy (usual care) before prescribing SLNB.
Methods: We identified T1 and T2 patients who underwent WLE and SLNB but not completion lymph node dissection between 2007 and 2018. Controls were T1 patients who only underwent WLE. Costs for WLE and SLNB were calculated by converting institutional cost data to standardized Medicare reimbursement rates. We then developed a decision tree to compare the cost of Merlin testing to that of a no-testing strategy (usual care).
Results: The average standardized cost of WLE was $2066, whereas the cost of WLE and SLNB was $11,976 based on Medicare rates. At a cost below $7350 for T1b melanoma and $4600 for T1b to T2 melanoma, Merlin testing was cost-saving compared to a no-testing strategy (usual care), assuming Medicare reimbursement rates.
Conclusion: Merlin testing for T1b and T2 melanoma is potentially cost saving depending on the cost of the molecular assay and SLNB reimbursement rates. In addition to being cost saving, Merlin is expected to improve health-related quality of life.
© 2022 The Authors. International Journal of Dermatology published by Wiley Periodicals LLC on behalf of the International Society of Dermatology.
Figures
Similar articles
-
Use and Costs of Sentinel Lymph Node Biopsy in Non-Ulcerated T1b Melanoma: Analysis of a Population-Based Registry.Ann Surg Oncol. 2021 Jul;28(7):3470-3478. doi: 10.1245/s10434-021-09998-6. Epub 2021 Apr 26. Ann Surg Oncol. 2021. PMID: 33900501
-
Accuracy of lymphatic mapping and sentinel lymph node biopsy after previous wide local excision in patients with primary melanoma.Cancer. 2006 Dec 1;107(11):2647-52. doi: 10.1002/cncr.22320. Cancer. 2006. PMID: 17063497
-
Is there a relation between type of primary melanoma treatment and the development of intralymphatic metastasis? A review of the literature.Cancer Treat Rev. 2016 Apr;45:120-8. doi: 10.1016/j.ctrv.2016.02.007. Epub 2016 Mar 2. Cancer Treat Rev. 2016. PMID: 27015564 Review.
-
Using the Merlin assay for reducing sentinel lymph node biopsy complications in melanoma: a retrospective cohort study.Int J Dermatol. 2022 Jul;61(7):848-854. doi: 10.1111/ijd.16056. Epub 2022 Jan 31. Int J Dermatol. 2022. PMID: 35100440 Free PMC article.
-
Sentinel lymph node risk prognostication in primary cutaneous melanoma through tissue-based profiling, potentially redefining the need for sentinel lymph node biopsy.Eur J Cancer. 2024 May;202:113989. doi: 10.1016/j.ejca.2024.113989. Epub 2024 Mar 8. Eur J Cancer. 2024. PMID: 38518535 Review.
Cited by
-
Risk Prediction Models for Sentinel Node Positivity in Melanoma: A Systematic Review and Meta-Analysis.JAMA Dermatol. 2025 May 1;161(5):523-532. doi: 10.1001/jamadermatol.2025.0113. JAMA Dermatol. 2025. PMID: 40072444
References
-
- Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384:72–9. - PubMed
-
- Weitemeyer MB, Helvind NM, Brinck AM, Hölmich LR, Chakera AH. More sentinel lymph node biopsies for thin melanomas after transition to AJCC 8th edition do not increase positivity rate: a Danish population‐based study of 7148 patients. J Surg Oncol. 2022;125:498–508. - PubMed
-
- Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2021;19:364–76. - PubMed
-
- Luke JJ, Rutkowski P, Queirolo P, del Vecchio M, Mackiewicz J, Chiarion‐Sileni V, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE‐716): a randomised, double‐blind, phase 3 trial. Lancet. 2022;399:1718–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical