Pre-emptive antifungal therapy versus empirical antifungal therapy for febrile neutropenia in people with cancer
- PMID: 36440894
- PMCID: PMC9703870
- DOI: 10.1002/14651858.CD013604.pub2
Pre-emptive antifungal therapy versus empirical antifungal therapy for febrile neutropenia in people with cancer
Abstract
Background: Intensive cytotoxic chemotherapy for people with cancer can cause severe and prolonged cytopenia, especially neutropenia, a critical condition that is potentially life-threatening. When manifested by fever and neutropenia, it is called febrile neutropenia (FN). Invasive fungal disease (IFD) is one of the serious aetiologies of chemotherapy-induced FN. In pre-emptive therapy, physicians only initiate antifungal therapy when an invasive fungal infection is detected by a diagnostic test. Compared to empirical antifungal therapy, pre-emptive therapy may reduce the use of antifungal agents and associated adverse effects, but may increase mortality. The benefits and harms associated with the two treatment strategies have yet to be determined. OBJECTIVES: To assess the relative efficacy, safety, and impact on antifungal agent use of pre-emptive versus empirical antifungal therapy in people with cancer who have febrile neutropenia.
Search methods: We searched CENTRAL, MEDLINE Ovid, Embase Ovid, and ClinicalTrials.gov to October 2021.
Selection criteria: We included randomised controlled trials (RCTs) that compared pre-emptive antifungal therapy with empirical antifungal therapy for people with cancer.
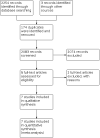
Data collection and analysis: We identified 2257 records from the databases and handsearching. After removing duplicates, screening titles and abstracts, and reviewing full-text reports, we included seven studies in the review. We evaluated the effects on all-cause mortality, mortality ascribed to fungal infection, proportion of antifungal agent use (other than prophylactic use), duration of antifungal use (days), invasive fungal infection detection, and adverse effects for the comparison of pre-emptive versus empirical antifungal therapy. We presented the overall certainty of the evidence for each outcome according to the GRADE approach.
Main results: This review includes 1480 participants from seven randomised controlled trials. Included studies only enroled participants at high risk of FN (e.g. people with haematological malignancy); none of them included participants at low risk (e.g. people with solid tumours). Low-certainty evidence suggests there may be little to no difference between pre-emptive and empirical antifungal treatment for all-cause mortality (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.72 to 1.30; absolute effect, reduced by 3/1000); and for mortality ascribed to fungal infection (RR 0.92, 95% CI 0.45 to 1.89; absolute effect, reduced by 2/1000). Pre-emptive therapy may decrease the proportion of antifungal agent used more than empirical therapy (other than prophylactic use; RR 0.71, 95% CI 0.47 to 1.05; absolute effect, reduced by 125/1000; very low-certainty evidence). Pre-emptive therapy may reduce the duration of antifungal use more than empirical treatment (mean difference (MD) -3.52 days, 95% CI -6.99 to -0.06, very low-certainty evidence). Pre-emptive therapy may increase invasive fungal infection detection compared to empirical treatment (RR 1.70, 95% CI 0.71 to 4.05; absolute effect, increased by 43/1000; very low-certainty evidence). Although we were unable to pool adverse events in a meta-analysis, there seemed to be no apparent difference in the frequency or severity of adverse events between groups. Due to the nature of the intervention, none of the seven RCTs could blind participants and personnel related to performance bias. We identified considerable clinical and statistical heterogeneity, which reduced the certainty of the evidence for each outcome. However, the two mortality outcomes had less statistical heterogeneity than other outcomes.
Authors' conclusions: For people with cancer who are at high-risk of febrile neutropenia, pre-emptive antifungal therapy may reduce the duration and rate of use of antifungal agents compared to empirical therapy, without increasing over-all and IFD-related mortality; but the evidence regarding invasive fungal infection detection and adverse events was inconsistent and uncertain.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Yu Uneno: none known Haruki Imura: none known Yosuke Makuuchi: has received lecture fees from Merck, Sharp and Dohme and Bristol‐Myers Squibb on topics outside the scope of this review. Kentaro Tochitani: none known Norio Watanabe: none known
Figures










Update of
- doi: 10.1002/14651858.CD013604
Similar articles
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD014963. doi: 10.1002/14651858.CD014963.pub2. Cochrane Database Syst Rev. 2022. PMID: 36385229 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
-
Acupuncture for treating overactive bladder in adults.Cochrane Database Syst Rev. 2022 Sep 23;9(9):CD013519. doi: 10.1002/14651858.CD013519.pub2. Cochrane Database Syst Rev. 2022. PMID: 36148895 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
Cited by
-
Coccidioidomycosis and Histoplasmosis in Immunocompetent Individuals: A Comprehensive Review of Clinical Features, Diagnosis, and Management.Cureus. 2024 Sep 1;16(9):e68375. doi: 10.7759/cureus.68375. eCollection 2024 Sep. Cureus. 2024. PMID: 39355457 Free PMC article. Review.
-
2024 update of the AGIHO guideline on diagnosis and empirical treatment of fever of unknown origin (FUO) in adult neutropenic patients with solid tumours and hematological malignancies.Lancet Reg Health Eur. 2025 Jan 31;51:101214. doi: 10.1016/j.lanepe.2025.101214. eCollection 2025 Apr. Lancet Reg Health Eur. 2025. PMID: 39973942 Free PMC article. Review.
-
Plants against cancer: the immune-boosting herbal microbiome: not of the plant, but in the plant. Basic concepts, introduction, and future resource for vaccine adjuvant discovery.Front Oncol. 2023 Jul 31;13:1180084. doi: 10.3389/fonc.2023.1180084. eCollection 2023. Front Oncol. 2023. PMID: 37588095 Free PMC article. Review.
-
Guidelines for Antibiotics Prescription in Critically Ill Patients.Indian J Crit Care Med. 2024 Aug;28(Suppl 2):S104-S216. doi: 10.5005/jp-journals-10071-24677. Epub 2024 Aug 10. Indian J Crit Care Med. 2024. PMID: 39234229 Free PMC article.
-
BALF metagenomic next-generation sequencing analysis in hematological malignancy patients with suspected pulmonary infection: clinical significance of negative results.Front Med (Lausanne). 2023 Jun 28;10:1195629. doi: 10.3389/fmed.2023.1195629. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37457591 Free PMC article.
References
References to studies included in this review
Aguado 2015 {published data only}
-
- Aguado JM, Vázquez L, Fernández-Ruiz M, Villaescusa T, Ruiz-Camps I, Barba P, et al, PCRAGA Study Group, Spanish Stem Cell Transplantation Group, Study Group of Medical Mycology of the Spanish Society of Clinical Microbiology and Infectious Diseases, Spanish Network for Research in Infectious Diseases. Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based Aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: a randomized controlled trial. Clinical Infectious Diseases 2015;60(3):405-14. [DOI: 10.1093/cid/ciu833] - DOI - PubMed
Blennow 2010 {published data only}
-
- Blennow O, Remberger M, Klingspor L, Omazic B, Fransson K, Ljungman P, et al. Randomized PCR-based therapy and risk factors for invasive fungal infection following reduced-intensity conditioning and hematopoietic SCT. Bone Marrow Transplant 2010;45(12):1710-8. [DOI: 10.1038/bmt.2010.38] - DOI - PubMed
Cordonnier 2009 {published data only}
-
- Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clinical Infectious Diseases 2009;48(8):1042-51. - PubMed
Hebart 2009 {published data only}
-
- Hebart H, Klingspor L, Klingebiel T, Loeffler J, Tollemar J, Ljungman P, et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant 2009;43(7):553-61. [DOI: 10.1038/bmt.2008.355] - DOI - PubMed
Morrissey 2013 {published data only}
-
- Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, et al, Australasian Leukaemia Lymphoma Group and the Australia and New Zealand Mycology Interest Group. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infectious Diseases 2013;13(6):519-28. [DOI: 10.1016/S1473-3099(13)70076-8] - DOI - PubMed
Tan 2011 {published data only}
-
- Tan BH, Low JG, Chlebicka NL, Kurup A, Cheah FK, Lin RT, et al. Galactomannan-guided preemptive vs. empirical antifungals in the persistently febrile neutropenic patient: a prospective randomized study. International Journal of Infectious Diseases 2011;15(5):e350-6. [DOI: 10.1016/j.ijid.2011.01.011] - DOI - PubMed
References to studies excluded from this review
Kanda 2020 {published data only}
-
- Kanda Y, Kimura SI, Iino M, Fukuda T, Sakaida E, Oyake T, et al, Japan Febrile Neutropenia Study Group. D-index-guided early antifungal therapy versus empiric antifungal therapy for persistent febrile neutropenia: a randomized controlled noninferiority trial. Journal of Clinical Oncology 2020 Mar 10;38(8):815-22. [DOI: 10.1200/JCO.19.01916] - DOI - PubMed
Additional references
Al‐Tawfiq 2019
-
- Al-Tawfiq JA, Hinedi K, Khairallah H, Saadeh B, Abbasi S, Noureen M, et al. Epidemiology and source of infection in patients with febrile neutropenia: a ten-year longitudinal study. Journal of Infection and Public Health 2019;12(3):364-6. - PubMed
Asano‐Mori 2008
-
- Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, et al. False-positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. Journal of Antimicrobial Chemotherapy 2008;61(2):411-6. - PubMed
Ascioglu 2002
-
- Ascioglu S, Rex JH, Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical Infectious Diseases 2002;34(1):7-14. - PubMed
Bow 2002
-
- Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer 2002;94(12):3230-46. - PubMed
Chan 2011
Chen 2017
Cho 1979
-
- Cho SY, Choi HY. Opportunistic fungal infection among cancer patients. A ten-year autopsy study. American Journal of Clinical Pathology 1979;72(4):617-21. - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed October 2021. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
De Pauw 2008
-
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008;46(12):1813-21. - PMC - PubMed
Donnelly 2020
-
- Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical Infectious Diseases 2020;71(6):1367-76. [DOI: 10.1093/cid/ciz1008] - DOI - PMC - PubMed
EORTC 1989
-
- EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. American Journal of Medicine 1989;86:668-72. - PubMed
Escuissato 2005
-
- Escuissato DL, Gasparetto EL, Marchiori E, Rocha Gde M, Inoue C, Pasquini R, et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. American Journal of Roentgenology 2005;185(3):608-15. - PubMed
Freifeld 2011
-
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2011;52(4):e56-93. - PubMed
Fung 2015
Glasmacher 2003
-
- Glasmacher A, Prentice A, Gorschluter M, Engelhart S, Hahn C, Djulbegovic B, et al. Itraconazole prevents invasive fungal infections in neutropenic patients treated for hematologic malignancies: evidence from a meta-analysis of 3,597 patients. Journal of Clinical Oncology 2003;21(24):4615-26. - PubMed
GRADEPro GDT [Computer program]
-
- GRADEpro GDT. Version Accessed October 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/PDF/v5.2/chapter-08.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. (updated February 2022) Cochrane, 2022 . Available from www.training.cochrane.org/handbook.
Klastersky 2004
-
- Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clinical Infectious Diseases 2004;39(Suppl 1):S32-7. - PubMed
Langendam 2013
Maddy 2019
-
- Maddy AJ, Sanchez N, Shukla BS, Maderal AD. Dermatological manifestations of fungal infection in patients with febrile neutropaenia: a review of the literature. Mycoses 2019;62(9):826-34. - PubMed
Maertens 2012
Maertens 2018
-
- Maertens JA, Girmenia C, Brüggemann RJ, Duarte RF, Kibbler CC, Ljungman P, et al, European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. Journal of Antimicrobial Chemotherapy 2018;73(12):3221-30. - PubMed
Marr 2005
-
- Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clinical Infectious Diseases 2005;40(12):1762-9. - PubMed
Meader 2014
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. - PubMed
National Cancer Institiute 2017
-
- Natinal Cancer Instittute. Common terminology criteria for adverse events (CTCAE) v5.0. Available at ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Nov 2017 (Last access date: 19 August 2022).
Pizzo 1982
-
- Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. American Journal of Medicine 1982;72(1):101-11. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Robenshtok 2007
-
- Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. Journal of Clinical Oncology 2007;25(34):5471-89. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH, on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Smith 2015
-
- Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. Journal of Clinical Oncology 2015;33(28):3199-212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

