Real-World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients
- PMID: 36441040
- PMCID: PMC9613796
- DOI: 10.1016/j.chest.2022.10.020
Real-World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients
Abstract
Background: Neutralizing monoclonal antibodies (mAbs) were authorized for the treatment of COVID-19 outpatients based on clinical trials completed early in the pandemic, which were underpowered for mortality and subgroup analyses. Real-world data studies are promising for further assessing rapidly deployed therapeutics.
Research question: Did mAb treatment prevent progression to severe disease and death across pandemic phases and based on risk factors, including prior vaccination status?
Study design and methods: This observational cohort study included nonhospitalized adult patients with SARS-CoV-2 infection from November 2020 to October 2021 using electronic health records from a statewide health system plus state-level vaccine and mortality data. Using propensity matching, we selected approximately 2.5 patients not receiving mAbs for each patient who received mAb treatment under emergency use authorization. The primary outcome was 28-day hospitalization; secondary outcomes included mortality and hospitalization severity.
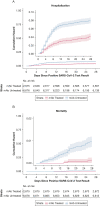
Results: Of 36,077 patients with SARS-CoV-2 infection, 2,675 receiving mAbs were matched to 6,677 patients not receiving mAbs. Compared with mAb-untreated patients, mAb-treated patients had lower all-cause hospitalization (4.0% vs 7.7%; adjusted OR, 0.48; 95% CI, 0.38-0.60) and all-cause mortality (0.1% vs 0.9%; adjusted OR, 0.11; 95% CI, 0.03-0.29) to day 28; differences persisted to day 90. Among hospitalized patients, mAb-treated patients had shorter hospital length of stay (5.8 vs 8.5 days) and lower risk of mechanical ventilation (4.6% vs 16.6%). Results were similar for preventing hospitalizations during the Delta variant phase (adjusted OR, 0.35; 95% CI, 0.25-0.50) and across subgroups. Number-needed-to-treat (NNT) to prevent hospitalization was lower for subgroups with higher baseline risk of hospitalization; for example, multiple comorbidities (NNT = 17) and not fully vaccinated (NNT = 24) vs no comorbidities (NNT = 88) and fully vaccinated (NNT = 81).
Interpretation: Real-world data revealed a strong association between receipt of mAbs and reduced hospitalization and deaths among COVID-19 outpatients across pandemic phases. Real-world data studies should be used to guide practice and policy decisions, including allocation of scarce resources.
Keywords: COVID-19; Delta variant; hospitalization; mechanical ventilation; monoclonal antibody; outpatient.
Copyright © 2022 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Johns Hopkins Coronavirus Resource Center United States cases by county. Johns Hopkins University & Medicine. 2020. https://coronavirus.jhu.edu/us-map
-
- Centers for Disease Control and Prevention Science Brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine... - PubMed
-
- COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

