A Push-Pull Mechanism Between PRRT2 and β4-subunit Differentially Regulates Membrane Exposure and Biophysical Properties of NaV1.2 Sodium Channels
- PMID: 36441479
- PMCID: PMC9899197
- DOI: 10.1007/s12035-022-03112-x
A Push-Pull Mechanism Between PRRT2 and β4-subunit Differentially Regulates Membrane Exposure and Biophysical Properties of NaV1.2 Sodium Channels
Abstract
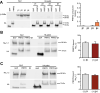
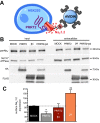
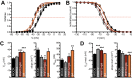
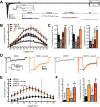
Proline-rich transmembrane protein 2 (PRRT2) is a neuron-specific protein implicated in the control of neurotransmitter release and neural network stability. Accordingly, PRRT2 loss-of-function mutations associate with pleiotropic paroxysmal neurological disorders, including paroxysmal kinesigenic dyskinesia, episodic ataxia, benign familial infantile seizures, and hemiplegic migraine. PRRT2 is a negative modulator of the membrane exposure and biophysical properties of Na+ channels NaV1.2/NaV1.6 predominantly expressed in brain glutamatergic neurons. NaV channels form complexes with β-subunits that facilitate the membrane targeting and the activation of the α-subunits. The opposite effects of PRRT2 and β-subunits on NaV channels raises the question of whether PRRT2 and β-subunits interact or compete for common binding sites on the α-subunit, generating Na+ channel complexes with distinct functional properties. Using a heterologous expression system, we have observed that β-subunits and PRRT2 do not interact with each other and act as independent non-competitive modulators of NaV1.2 channel trafficking and biophysical properties. PRRT2 antagonizes the β4-induced increase in expression and functional activation of the transient and persistent NaV1.2 currents, without affecting resurgent current. The data indicate that β4-subunit and PRRT2 form a push-pull system that finely tunes the membrane expression and function of NaV channels and the intrinsic neuronal excitability.
Keywords: Intrinsic excitability; Persistent Na+ current; Proline-rich transmembrane protein 2; Resurgent Na+ current; Transient Na+ current.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

