Efficacy and Safety of Nirmatrelvir/Ritonavir, Molnupiravir, and Remdesivir in a Real-World Cohort of Outpatients with COVID-19 at High Risk of Progression: The PISA Outpatient Clinic Experience
- PMID: 36441485
- PMCID: PMC9707131
- DOI: 10.1007/s40121-022-00729-2
Efficacy and Safety of Nirmatrelvir/Ritonavir, Molnupiravir, and Remdesivir in a Real-World Cohort of Outpatients with COVID-19 at High Risk of Progression: The PISA Outpatient Clinic Experience
Abstract
Introduction: Different antivirals are available for the treatment of outpatients with COVID-19. Our aim was to describe a real-world experience of outpatient management of COVID-19 subjects at high risk of progression.
Methods: This prospective observational study conducted in the University Hospital of Pisa (January 2022-July 2022) included consecutive COVID-19 outpatients with at least one risk factor for disease progression. Patients received nirmatrelvir/ritonavir, molnupiravir, or 3-day remdesivir, according to the Italian Medicines Agency (AIFA) indications. All patients were followed up until 30 days from the first positive nasopharyngeal swab. The primary endpoint was a composite of death or hospitalization. Secondary endpoints were occurrence of adverse events and a negative test within 10 days from the first positive test. Multivariable analysis was performed to identify factors associated with death or hospitalization.
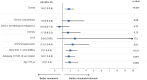
Results: Overall, 562 outpatients were included: 114 (20.3%) received molnupiravir, 252 (44.8%) nirmatrelvir/ritonavir, and 196 (34.9%) 3-day remdesivir. The composite endpoint occurred in 2.5% of patients and was more frequent in patients treated with remdesivir (5.1%) compared with molnupiravir (1.8%) or nirmatrelvir/ritonavir (0.8%, ANOVA among groups p = 0.012). On multivariable Cox regression analysis, presence of ≥ 3 comorbidities, hematological disease, gastrointestinal symptoms, and each-day increment from symptoms onset were factors associated with death or hospitalization, while antiviral treatment was not a predictor. Adverse events occurred more frequently in the nirmatrelvir/ritonavir group (49.2%). Nirmatrelvir/ritonavir compared with remdesivir was associated with a higher probability of having a negative test within 10 days from the first positive one.
Conclusion: Death or hospitalization did not differ among high-risk COVID-19 outpatients treated with currently available antivirals. Safety and time to a negative test differed among the three drugs.
Keywords: COVID-19; Molnupiravir; Nirmatrelvir/ritonavir; Progression; Remdesivir; SARS-CoV-2.
© 2022. The Author(s).
Figures
References
-
- Tiseo G, Yahav D, Paul M, Tinelli M, Gavazzi G, Mussini C, et al. What have we learned from the first to the second wave of COVID-19 pandemic? An international survey from the ESCMID Study Group for Infection in the Elderly (ESGIE) group. Eur J Clin Microbiol Infect Dis. 2022;41:281–288. doi: 10.1007/s10096-021-04377-1. - DOI - PMC - PubMed
-
- Menichetti F, Popoli P, Puopolo M, Spila Alegiani S, Tiseo G, et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4:e2136246. doi: 10.1001/jamanetworkopen.2021.36246. - DOI - PMC - PubMed
-
- Falcone M, Tiseo G, Valoriani B, Barbieri C, Occhineri S, Mazzetti P, et al. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther. 2021;10:2479–2488. doi: 10.1007/s40121-021-00525-4. - DOI - PMC - PubMed
-
- Yamasoba D, Kosugi Y, Kimura I, Fujita S, Uriu K, Ito J, Sato K, Genotype to Phenotype Japan (G2P-Japan) Consortium Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect Dis. 2022;22:942–943. doi: 10.1016/S1473-3099(22)00365-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

