First Randomized, Multicenter, Placebo-Controlled Study of Self-Administered Intranasal Etripamil for Acute Conversion of Spontaneous Paroxysmal Supraventricular Tachycardia (NODE-301)
- PMID: 36441560
- PMCID: PMC9760458
- DOI: 10.1161/CIRCEP.122.010915
First Randomized, Multicenter, Placebo-Controlled Study of Self-Administered Intranasal Etripamil for Acute Conversion of Spontaneous Paroxysmal Supraventricular Tachycardia (NODE-301)
Abstract
Background: Pharmacologic termination of paroxysmal supraventricular tachycardia (PSVT) often requires medically supervised intervention. Intranasal etripamil, is an investigational fast-acting, nondihydropyridine, L-type calcium channel blocker, designed for unsupervised self-administration to terminate atrioventricular nodal-dependent PSVT. Phase 2 results showed potential safety and efficacy of etripamil in 104 patients with PSVT.
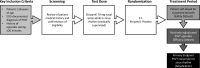
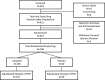
Methods: NODE-301, a phase 3, multicenter, double-blind, placebo-controlled study evaluated the efficacy and safety of etripamil nasal spray administered, unsupervised in patients with symptomatic sustained PSVT. After a medically supervised etripamil test dose while in sinus rhythm, patients were randomized 2:1 to receive etripamil 70 mg or placebo. When PSVT symptoms developed, patients applied a cardiac monitor and attempted a vagal maneuver; if symptoms persisted, they self-administered blinded treatment. An independent Adjudication Committee reviewed continuous electrocardiogram recordings. The primary efficacy endpoint was termination of adjudicated PSVT within 5 hours after study drug administration.
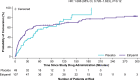
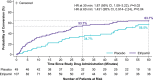
Results: NODE-301 accrued 156 positively adjudicated PSVT events treated with etripamil (n=107) or placebo (n=49). The hazard ratio for the primary endpoint, time-to-conversion to sinus rhythm during the 5-hour observation period, was 1.086 (95% CI, 0.726-1.623; P=0.12). In predefined sensitivity analyses, etripamil effects (compared with placebo) occurred at 3, 5, 10, 20, and 30 minutes (P<0.05). For example, at 30 minutes, there was a 53.7% of SVT conversion in the treatment arm compared to 34.7% in the placebo arm (hazard ratio, 1.87 [95% CI, 1.09-3.22]; P=0.02). Etripamil was well tolerated; adverse events were mainly related to transient nasal discomfort and congestion (19.6% and 8.0%, respectively, of randomized treatment-emergent adverse events.
Conclusions: Although the primary 5-hour efficacy endpoint was not met, analyses at earlier time points indicated an etripamil treatment effect in terminating PSVT. Etripamil self-administration during PSVT was safe and well tolerated. These results support continued clinical development of etripamil nasal spray for self-administration during PSVT in a medically unsupervised setting.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT03464019.
Keywords: atrioventricular node; blood pressure; calcium; etripamil; tachycardia; verapamil.
Figures
References
-
- Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller G-P, et al. ; ESC Scientific Document Group. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC) [published correction appears in Eur Heart J. 2020;41:4258]. Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467 - PubMed
-
- Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes MI, III, Field ME, Goldberger ZD, Hammill SC, et al. . 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2016;134:e232–233]. Circulation. 2016;133:e471–e505. doi: 10.1161/cir.0000000000000310 - PubMed
-
- Issa ZF, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2012.
-
- Ferguson JD, DiMarco JP. Contemporary management of paroxysmal supraventricular tachycardia. Circulation. 2003;107:1096–1099. doi: 10.1161/01.cir.0000059743.36226.e8 - PubMed
-
- Hamer A, Peter T, Mandel W. Atrioventricular node reentry: intravenous verapamil as a method of defining multiple electrophysiologic types. Am Heart J. 1983;105:629–642. doi: 10.1016/0002-8703(83)90488-x - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

