[18F]FluorThanatrace ([18F]FTT) PET Imaging of PARP-Inhibitor Drug-Target Engagement as a Biomarker of Response in Ovarian Cancer, a Pilot Study
- PMID: 36441795
- PMCID: PMC12012890
- DOI: 10.1158/1078-0432.CCR-22-1602
[18F]FluorThanatrace ([18F]FTT) PET Imaging of PARP-Inhibitor Drug-Target Engagement as a Biomarker of Response in Ovarian Cancer, a Pilot Study
Abstract
Purpose: PARP inhibitors have become the standard-of-care treatment for homologous recombination deficient (HRD) high-grade serous ovarian cancer (HGSOC). However, not all HRD tumors respond to PARPi. Biomarkers to predict response are needed. [18F]FluorThanatrace ([18F]FTT) is a PARPi-analog PET radiotracer that noninvasively measures PARP-1 expression. Herein, we evaluate [18F]FTT as a biomarker to predict response to PARPi in patient-derived xenograft (PDX) models and subjects with HRD HGSOC.
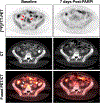
Experimental design: In PDX models, [18F]FTT-PET was performed before and after PARPi (olaparib), ataxia-telangiectasia inhibitor (ATRi), or both (PARPi-ATRi). Changes in [18F]FTT were correlated with tumor volume changes. Subjects were imaged with [18F]FTT-PET at baseline and after ∼1 week of PARPi. Changes in [18F]FTT-PET uptake were compared with changes in tumor size (RECISTv1.1), CA-125, and progression-free survival (PFS).
Results: A decrease in [18F]FTT tumor uptake after PARPi correlated with response to PARPi, or PARPi-ATRi treatment in PARPi-resistant PDX models (r = 0.77-0.81). In subjects (n = 11), percent difference in [18F]FTT-PET after ∼7 days of PARPi compared with baseline correlated with best RECIST response (P = 0.01), best CA-125 response (P = 0.033), and PFS (P = 0.027). All subjects with >50% reduction in [18F]FTT uptake had >6-month PFS and >50% reduction in CA-125. Utilizing only baseline [18F]FTT uptake did not predict such responses.
Conclusions: The decline in [18F]FTT uptake shortly after PARPi initiation provides a measure of drug-target engagement and shows promise as a biomarker to guide PARPi therapies in this pilot study. These results support additional preclinical mechanistic and clinical studies in subjects receiving PARPi ± combination therapy. See related commentary by Liu and Zamarin, p. 1384.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures




Comment in
-
Shedding Light on PARP Inhibitor Response through Functional Imaging.Clin Cancer Res. 2023 Apr 14;29(8):1384-1386. doi: 10.1158/1078-0432.CCR-22-3711. Clin Cancer Res. 2023. PMID: 36700799 Free PMC article.
Comment on
-
Shedding Light on PARP Inhibitor Response through Functional Imaging.Clin Cancer Res. 2023 Apr 14;29(8):1384-1386. doi: 10.1158/1078-0432.CCR-22-3711. Clin Cancer Res. 2023. PMID: 36700799 Free PMC article.
References
-
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495–505 - PubMed
-
- Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2021;22:620–31 - PubMed
-
- Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

