Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia
- PMID: 36441905
- PMCID: PMC10172873
- DOI: 10.1182/bloodadvances.2022009010
Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia
Abstract
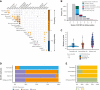
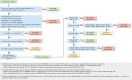
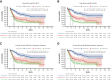
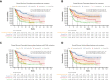
Risk stratification in acute myeloid leukemia (AML) remains principle in survival prognostication and treatment selection. The 2022 European LeukemiaNet (ELN) recommendations were recently published, with notable updates to risk group assignment. The complexity of risk stratification and comparative outcomes between the 2022 and 2017 ELN guidelines remains unknown. This comparative analysis evaluated outcomes between the 2017 and 2022 ELN criteria in patients enrolled within the multicenter Beat AML cohort. Five hundred thirteen patients were included. Most patients had 1 or 2 ELN risk-defining abnormalities. In patients with ≥2 ELN risk-defining mutations, 44% (n = 132) had mutations spanning multiple ELN risk categories. Compared with ELN 2017 criteria, the updated ELN 2022 guidelines changed the assigned risk group in 15% of patients, including 10%, 26%, and 6% of patients categorized as being at ELN 2017 favorable-, intermediate-, and adverse-risk, respectively. The median overall survival across ELN 2022 favorable-, intermediate-, and adverse-risk groups was not reached, 16.8, and 9.7 months, respectively. The ELN 2022 guidelines more accurately stratified survival between patients with intermediate- or adverse-risk AML treated with induction chemotherapy compared with ELN 2017 guidelines. The updated ELN 2022 guidelines better stratify survival between patients with intermediate- or adverse-risk AML treated with induction chemotherapy. The increased complexity of risk stratification with inclusion of additional cytogenetic and molecular aberrations necessitates clinical workflows simplifying risk stratification.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.G. serves on the advisory boards for Gamida Cell, Talaris Therapeutics. J.W.T. has received research support from Acerta, Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Kronos, Meryx, Petra, Schrodinger, Seattle Genetics, Syros, Takeda, and Tolero and serves on the advisory board for Recludix Pharma. B.J.D. reports serving on the advisory boards for Adela Bio, Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Celgene, DNA SEQ, Nemucore Medical Innovations, Novartis, RUNX1 Research Program, and Vivid Biosciences (inactive); the advisory board and stock interests in Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, GRAIL, and Recludix Pharma; the board of directors and stock interests in Amgen and Vincerx Pharma; the board of directors of Burroughs Wellcome Fund and CureOne; the joint steering committee for Beat AML LLS; advisory committee for Multicancer Early Detection Consortium; being the founder of VB Therapeutics; having a sponsored research agreement with Enliven Therapeutics and Recludix Pharma; receiving clinical trial funding from Novartis and AstraZeneca and royalties from patent 6958335 (Novartis exclusive license), OHSU, and Dana-Farber Cancer Institute (1 Merck exclusive license, 1 CytoImage, Inc exclusive license, and 1 Sun Pharma Advanced Research Company nonexclusive license); and holding US patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, and 11049247. R.S. serves as a consultant for COTA HealthCare. J.L. reports consulting for Takeda, Adaptive, Pfizer, Amgen, AbbVie, and KiTE; and receiving honoraria from Adaptive. E.T. has served on advisory boards for Astellas, AbbVie, Daiichi-Sankyo, and Servier and receives research funding from Incyte, Schrodinger, and AstraZeneca. The remaining authors declare no competing financial interests.
Figures

References
-
- Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441–2448. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

