UV Photoelectron Spectroscopy of Aqueous Solutions
- PMID: 36442085
- PMCID: PMC9774667
- DOI: 10.1021/acs.accounts.2c00523
UV Photoelectron Spectroscopy of Aqueous Solutions
Abstract
Knowledge of the electronic structure of an aqueous solution is a prerequisite to understanding its chemical and biological reactivity and its response to light. One of the most direct ways of determining electronic structure is to use photoelectron spectroscopy to measure electron binding energies. Initially, photoelectron spectroscopy was restricted to the gas or solid phases due to the requirement for high vacuum to minimize inelastic scattering of the emitted electrons. The introduction of liquid-jets and their combination with intense X-ray sources at synchrotrons in the late 1990s expanded the scope of photoelectron spectroscopy to include liquids. Liquid-jet photoelectron spectroscopy is now an active research field involving a growing number of research groups. A limitation of X-ray photoelectron spectroscopy of aqueous solutions is the requirement to use solutes with reasonably high concentrations in order to obtain photoelectron spectra with adequate signal-to-noise after subtracting the spectrum of water. This has excluded most studies of organic molecules, which tend to be only weakly soluble. A solution to this problem is to use resonance-enhanced photoelectron spectroscopy with ultraviolet (UV) light pulses (hν ≲ 6 eV). However, the development of UV liquid-jet photoelectron spectroscopy has been hampered by a lack of quantitative understanding of inelastic scattering of low kinetic energy electrons (≲5 eV) and the impact on spectral lineshapes and positions.In this Account, we describe the key steps involved in the measurement of UV photoelectron spectra of aqueous solutions: photoionization/detachment, electron transport of low kinetic energy electrons through the conduction band, transmission through the water-vacuum interface, and transport through the spectrometer. We also explain the steps we take to record accurate UV photoelectron spectra of liquids with excellent signal-to-noise. We then describe how we have combined Monte Carlo simulations of electron scattering and spectral inversion with molecular dynamics simulations of depth profiles of organic solutes in aqueous solution to develop an efficient and widely applicable method for retrieving true UV photoelectron spectra of aqueous solutions. The huge potential of our experimental and spectral retrieval methods is illustrated using three examples. The first is a measurement of the vertical detachment energy of the green fluorescent protein chromophore, a sparingly soluble organic anion whose electronic structure underpins its fluorescence and photooxidation properties. The second is a measurement of the vertical ionization energy of liquid water, which has been the subject of discussion since the first X-ray photoelectron spectroscopy measurement in 1997. The third is a UV photoelectron spectroscopy study of the vertical ionization energy of aqueous phenol which demonstrates the possibility of retrieving true photoelectron spectra from measurements with contributions from components with different concentration profiles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
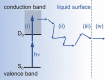
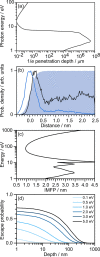






References
-
- Tau O.; Henley A.; Boichenko A. N.; Kleshchina N. N.; Riley R.; Wang B.; Winning D.; Lewin R.; Ward J.; Parkin I. P.; Hailes H. C.; Bochenkova A. V.; Fielding H. H. Liquid-microjet photoelectron spectroscopy of the green fluorescent protein chromophore. Nat. Commun. 2022, 13, 507.10.1038/s41467-022-28155-5. - DOI - PMC - PubMed
-
- Henley A.; Fielding H. H. Anion photoelectron spectroscopy of protein chromophores. Int. Rev. Phys. Chem. 2019, 38, 1–34. 10.1080/0144235X.2018.1548807. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

