Early diagnosis and treatment of Alzheimer's disease by targeting toxic soluble Aβ oligomers
- PMID: 36442093
- PMCID: PMC9894226
- DOI: 10.1073/pnas.2210766119
Early diagnosis and treatment of Alzheimer's disease by targeting toxic soluble Aβ oligomers
Abstract
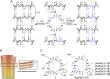
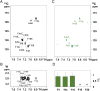
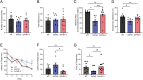
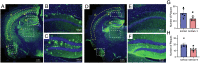
Transient soluble oligomers of amyloid-β (Aβ) are toxic and accumulate early prior to insoluble plaque formation and cognitive impairment in Alzheimer's disease (AD). Synthetic cyclic D,L-α-peptides (e.g., 1) self-assemble into cross β-sheet nanotubes, react with early Aβ species (1-3 mers), and inhibit Aβ aggregation and toxicity in stoichiometric concentrations, in vitro. Employing a semicarbazide as an aza-glycine residue with an extra hydrogen-bond donor to tune nanotube assembly and amyloid engagement, [azaGly6]-1 inhibited Aβ aggregation and toxicity at substoichiometric concentrations. High-resolution NMR studies revealed dynamic interactions between [azaGly6]-1 and Aβ42 residues F19 and F20, which are pivotal for early dimerization and aggregation. In an AD mouse model, brain positron emission tomography (PET) imaging using stable 64Cu-labeled (aza)peptide tracers gave unprecedented early amyloid detection in 44-d presymptomatic animals. No tracer accumulation was detected in the cortex and hippocampus of 44-d-old 5xFAD mice; instead, intense PET signal was observed in the thalamus, from where Aβ oligomers may spread to other brain parts with disease progression. Compared with standard 11C-labeled Pittsburgh compound-B (11C-PIB), which binds specifically fibrillar Aβ plaques, 64Cu-labeled (aza)peptide gave superior contrast and uptake in young mouse brain correlating with Aβ oligomer levels. Effectively crossing the blood-brain barrier (BBB), peptide 1 and [azaGly6]-1 reduced Aβ oligomer levels, prolonged lifespan of AD transgenic Caenorhabditis elegans, and abated memory and behavioral deficits in nematode and murine AD models. Cyclic (aza)peptides offer novel promise for early AD diagnosis and therapy.
Keywords: Alzheimer’s disease; PET imaging; cyclic D,L-α-(aza)peptide; early diagnosis and therapy; soluble Aβ oligomers.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Hamley I. W., The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 112, 5147–5192 (2012). - PubMed
-
- Chiti F., Dobson C. M., Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006). - PubMed
-
- Stefani M., Dobson C. M., Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 81, 678–699 (2003). - PubMed
-
- Hardy J., Selkoe D. J., The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

