Brain PET Imaging: Frontotemporal Dementia
- PMID: 36442960
- PMCID: PMC9884902
- DOI: 10.1016/j.cpet.2022.09.010
Brain PET Imaging: Frontotemporal Dementia
Abstract
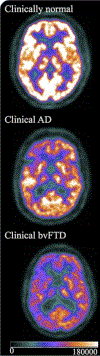
Brain PET adds value in diagnosing neurodegenerative disorders, especially frontotemporal dementia (FTD) due to its syndromic presentation that overlaps with a variety of other neurodegenerative and psychiatric disorders. 18F-FDG-PET has improved sensitivity and specificity compared with structural MR imaging, with optimal diagnostic results achieved when both techniques are utilized. PET demonstrates superior sensitivity compared with SPECT for FTD diagnosis that is primarily a supplement to other imaging and clinical evaluations. Tau-PET and amyloid-PET primary use in FTD diagnosis is differentiation from Alzheimer disease, although these methods are limited mainly to research settings.
Keywords: (18)F-FDG-PET; Alzheimer disease (AD); Amyloid-PET; Frontotemporal dementia (FTD); MRI; Neurodegenerative disorders fused-in-sarcoma protein (FUS); SPECT; Tau-PET.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures




References
-
- Olney RK, Murphy J, Forshew D, et al. The effects of executive and behavioral dysfunction on the course of ALS. Neurology 2005;65(11):1774–7. - PubMed
-
- Duignan JA, Haughey A, Kinsella JA, et al. Molecular and anatomical imaging of dementia with Lewy bodies and frontotemporal lobar degeneration. Semin Nucl Med 2021;51(3):264–74. - PubMed
-
- Uber die Beziehungen der senilen Hirnatrophie zur Aphasie | CiNii Research. Available at: https://cir.nii.ac.jp/crid/1572261550500971008. Accessed June 8, 2022.
-
- Alzheimer A ü ber eigenartige Krankheitsfä lle des spä teren Alters. Z fü r die gesamte Neurologie Psychiatrie 1911;4(1):356–85.
-
- Thibodeau MP, Miller BL. Limits and current knowledge of Pick’s disease: its differential diagnosis”. A translation of the 1957 Delay, Brion, Escourolle article. Neurocase 2013;19(5):417–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

