Sounding the Alarm: Sex Differences in Rat Ultrasonic Vocalizations during Pavlovian Fear Conditioning and Extinction
- PMID: 36443006
- PMCID: PMC9797209
- DOI: 10.1523/ENEURO.0382-22.2022
Sounding the Alarm: Sex Differences in Rat Ultrasonic Vocalizations during Pavlovian Fear Conditioning and Extinction
Abstract
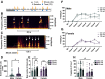
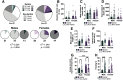
Pavlovian fear conditioning is a prevalent tool in the study of aversive learning, which is a key component of stress-related psychiatric disorders. Adult rats can exhibit various threat-related behaviors, including freezing, motor responses, and ultrasonic vocalizations (USVs). While these responses can all signal aversion, we know little about how they relate to one another. Here we characterize USVs emitted by male and female rats during cued fear acquisition and extinction, and assess the relationship between different threat-related behaviors. We found that males consistently emitted >22 kHz calls (referred to here as "alarm calls") than females, and that alarm call frequency in males, but not females, related to the intensity of the shock stimulus. Interestingly, 25% of males and 45% of females did not emit any alarm calls at all. Males that did make alarm calls had significantly higher levels of freezing than males who did not, while no differences in freezing were observed between female Alarm callers and Non-alarm callers. Alarm call emission was also affected by the predictability of the shock; when unpaired from a tone cue, both males and females started emitting alarm calls significantly later. During extinction learning and retrieval sessions, males were again more likely than females to emit alarm calls, which followed an extinction-like reduction in frequency. Collectively these data suggest sex dependence in how behavioral readouts relate to innate and conditioned threat responses. Importantly, we suggest that the same behaviors can signal sex-dependent features of aversion.
Keywords: SABV; defensive behaviors; fear conditioning; ultrasonic vocalizations.
Copyright © 2022 Laine et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
ChAT::Cre transgenic rats show sex-dependent altered fear behaviors, ultrasonic vocalizations and cholinergic marker expression.Genes Brain Behav. 2023 Feb;22(1):e12837. doi: 10.1111/gbb.12837. Epub 2023 Jan 13. Genes Brain Behav. 2023. PMID: 36636833 Free PMC article.
-
Individual Differences in Conditioned Fear and Extinction in Female Rats.Front Behav Neurosci. 2021 Aug 18;15:740313. doi: 10.3389/fnbeh.2021.740313. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34489657 Free PMC article.
-
Male rats emit aversive 44-kHz ultrasonic vocalizations during prolonged Pavlovian fear conditioning.Elife. 2024 Dec 10;12:RP88810. doi: 10.7554/eLife.88810. Elife. 2024. PMID: 39656518 Free PMC article.
-
Rat 22kHz ultrasonic vocalizations as alarm cries.Behav Brain Res. 2007 Sep 4;182(2):166-72. doi: 10.1016/j.bbr.2006.11.038. Epub 2006 Dec 14. Behav Brain Res. 2007. PMID: 17173984 Review.
-
Types and functions of ultrasonic vocalizations in laboratory rats and mice.J Am Assoc Lab Anim Sci. 2007 Jan;46(1):28-34. J Am Assoc Lab Anim Sci. 2007. PMID: 17203913 Review.
Cited by
-
Comparing behaviours induced by natural memory retrieval and optogenetic reactivation of an engram ensemble in mice.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230227. doi: 10.1098/rstb.2023.0227. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853560 Free PMC article.
-
CB1R blockade unmasks TRPV1-mediated contextual fear generalization in female, but not male rats.Neuropsychopharmacology. 2023 Sep;48(10):1500-1508. doi: 10.1038/s41386-023-01650-z. Epub 2023 Jul 17. Neuropsychopharmacology. 2023. PMID: 37460772 Free PMC article.
-
Contributions of associative and non-associative learning to the dynamics of defensive ethograms.bioRxiv [Preprint]. 2024 Sep 19:2023.07.06.547975. doi: 10.1101/2023.07.06.547975. bioRxiv. 2024. Update in: Elife. 2024 Dec 16;12:RP90414. doi: 10.7554/eLife.90414. PMID: 39345429 Free PMC article. Updated. Preprint.
-
Effects of sex and estrous cycle on action-outcome contingencies.Behav Brain Res. 2025 Feb 4;477:115317. doi: 10.1016/j.bbr.2024.115317. Epub 2024 Oct 28. Behav Brain Res. 2025. PMID: 39490537
-
Behavioral and neural correlates of diverse conditioned fear responses in male and female rats.Neurobiol Stress. 2024 Sep 21;33:100675. doi: 10.1016/j.ynstr.2024.100675. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39391589 Free PMC article.
References
-
- Blanchard RJ, Flannelly KJ, Blanchard DC (1986) Defensive behavior of laboratory and wild Rattus norvegicus. J Comp Psychol 100:101–107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
