Loss of function of CMPK2 causes mitochondria deficiency and brain calcification
- PMID: 36443312
- PMCID: PMC9705363
- DOI: 10.1038/s41421-022-00475-2
Loss of function of CMPK2 causes mitochondria deficiency and brain calcification
Abstract
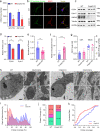
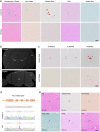
Brain calcification is a critical aging-associated pathology and can cause multifaceted neurological symptoms. Cerebral phosphate homeostasis dysregulation, blood-brain barrier defects, and immune dysregulation have been implicated as major pathological processes in familial brain calcification (FBC). Here, we analyzed two brain calcification families and identified calcification co-segregated biallelic variants in the CMPK2 gene that disrupt mitochondrial functions. Transcriptome analysis of peripheral blood mononuclear cells (PBMCs) isolated from these patients showed impaired mitochondria-associated metabolism pathways. In situ hybridization and single-cell RNA sequencing revealed robust Cmpk2 expression in neurons and vascular endothelial cells (vECs), two cell types with high energy expenditure in the brain. The neurons in Cmpk2-knockout (KO) mice have fewer mitochondrial DNA copies, down-regulated mitochondrial proteins, reduced ATP production, and elevated intracellular inorganic phosphate (Pi) level, recapitulating the mitochondrial dysfunction observed in the PBMCs isolated from the FBC patients. Morphologically, the cristae architecture of the Cmpk2-KO murine neurons was also impaired. Notably, calcification developed in a progressive manner in the homozygous Cmpk2-KO mice thalamus region as well as in the Cmpk2-knock-in mice bearing the patient mutation, thus phenocopying the calcification pathology observed in the patients. Together, our study identifies biallelic variants of CMPK2 as novel genetic factors for FBC; and demonstrates how CMPK2 deficiency alters mitochondrial structures and functions, thereby highlighting the mitochondria dysregulation as a critical pathogenic mechanism underlying brain calcification.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
- U1905210/National Natural Science Foundation of China (National Science Foundation of China)
- 82025012/National Natural Science Foundation of China (National Science Foundation of China)
- 81901158/National Natural Science Foundation of China (National Science Foundation of China)
- 82171841/National Natural Science Foundation of China (National Science Foundation of China)
- 81901324/National Natural Science Foundation of China (National Science Foundation of China)
- 81870895/National Natural Science Foundation of China (National Science Foundation of China)
- 31771139/National Natural Science Foundation of China (National Science Foundation of China)
- 2019J02010/Natural Science Foundation of Fujian Province (Fujian Provincial Natural Science Foundation)
- 2021J01221/Natural Science Foundation of Fujian Province (Fujian Provincial Natural Science Foundation)
- 2022M710704/China Postdoctoral Science Foundation
- 2021M693246/China Postdoctoral Science Foundation
- ZR2019MH060/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
- QYZDJ-SSW-SMC010/Chinese Academy of Sciences (CAS)
- 16JC1420202/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

